U.S. health regulators authorized the use of Novavax’s COVID-19 vaccine, providing a new option that works differently than the two leading vaccines based on mRNA technology, but the move comes at a time when uptake of shots and boosters is low.
U.S. health regulators authorized the use of Novavax Inc.’s COVID-19 vaccine, providing a new option that works differently than the two leading vaccines, both based on mRNA technology, but the move comes at a time when uptake of shots and boosters is low.
However, the U.S. vaccine program has slowed to a crawl with the numbers getting either primary shots or booster doses remaining static for weeks, based on data from the CDC . The Novavax shot is aimed at the original strain of the virus, which has quickly been replaced by newer variants, including omicron and its many subvariants, which are now dominant. Moderna and Pfizer are working to develop vaccines specifically targeting omicron.The World Health Organization said Thursday that BA.
See: Pandemic is still a public health emergency, warns WHO, urging greater surveillance including testing and sequencing Coronavirus Update: MarketWatch’s daily roundup has been curating and reporting all the latest developments every weekday since the coronavirus pandemic began• A labor shortage in the U.K. has been exacerbated by the number of people who are laid low after getting COVID, according to a new report. The report, published on the Taylor & Francis Online site, looked at the impact of long COVID on the workforce.
Canada Latest News, Canada Headlines
Similar News:You can also read news stories similar to this one that we have collected from other news sources.
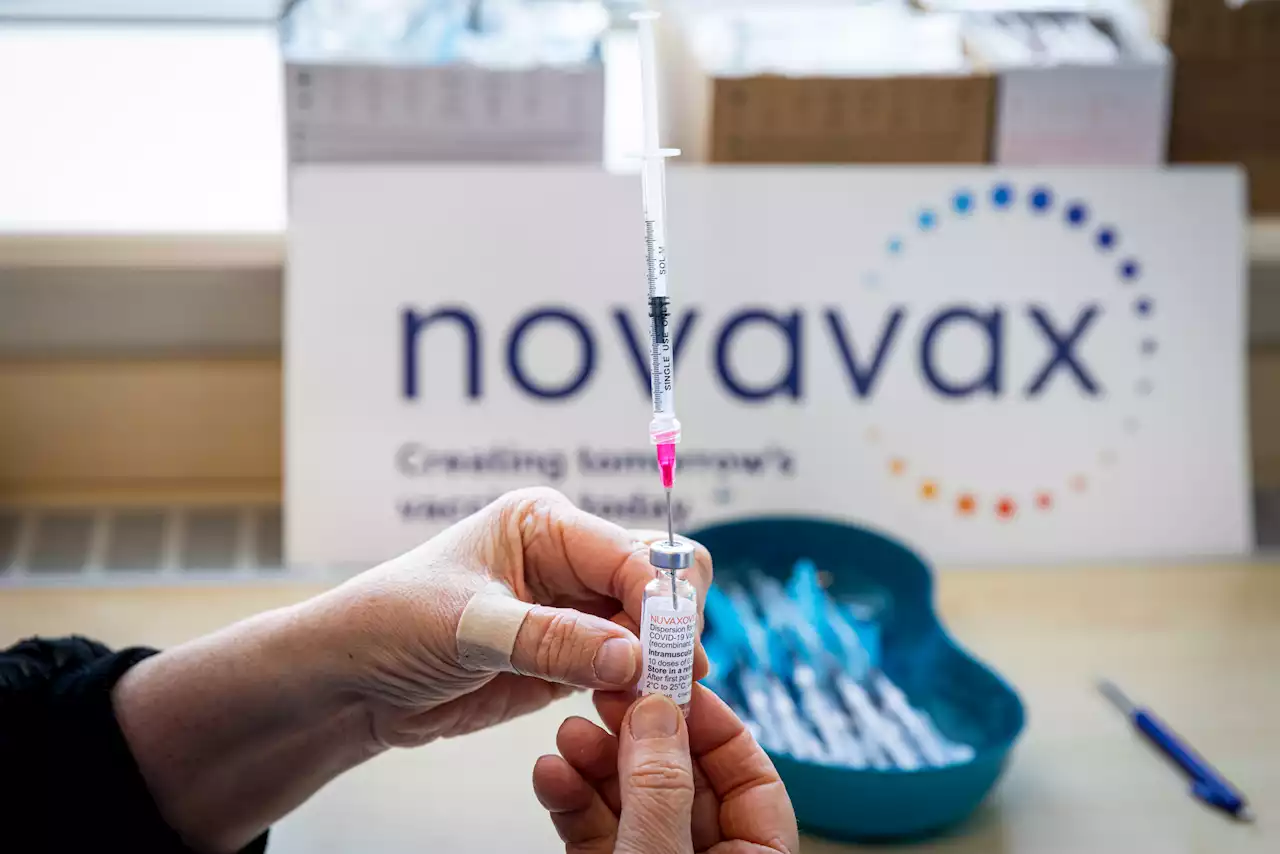 Novavax explained: how the COVID-19 vaccine differs from previous shotsThe Novavax vaccine has seen less fanfare as it moves through the regulatory process. But it could play an important role in the pandemic.
Novavax explained: how the COVID-19 vaccine differs from previous shotsThe Novavax vaccine has seen less fanfare as it moves through the regulatory process. But it could play an important role in the pandemic.
Read more »
 US regulators OK new COVID-19 shot option from NovavaxNovavax makes a more traditional type of shot than the three other COVID-19 vaccines available for use in the U.S.
US regulators OK new COVID-19 shot option from NovavaxNovavax makes a more traditional type of shot than the three other COVID-19 vaccines available for use in the U.S.
Read more »
 US regulators OK new COVID-19 shot option from NovavaxNovavax makes a more traditional type of shot than the three other COVID-19 vaccines available for use in the U.S.
US regulators OK new COVID-19 shot option from NovavaxNovavax makes a more traditional type of shot than the three other COVID-19 vaccines available for use in the U.S.
Read more »
 US regulators OK new COVID-19 shot option from NovavaxThe U.S. is getting another COVID-19 vaccine choice
US regulators OK new COVID-19 shot option from NovavaxThe U.S. is getting another COVID-19 vaccine choice
Read more »
 U.S. health officials approve new COVID-19 shot from NovavaxThe U.S. is getting another COVID-19 vaccine choice as the Food and Drug Administration on Wednesday cleared Novavax shots for adults. Novavax makes a more...
U.S. health officials approve new COVID-19 shot from NovavaxThe U.S. is getting another COVID-19 vaccine choice as the Food and Drug Administration on Wednesday cleared Novavax shots for adults. Novavax makes a more...
Read more »
 FDA authorizes Novavax COVID-19 vaccineThe FDA on Wednesday issued an emergency use authorization for Novavax's COVID-19 vaccine for adults 18 and older.
FDA authorizes Novavax COVID-19 vaccineThe FDA on Wednesday issued an emergency use authorization for Novavax's COVID-19 vaccine for adults 18 and older.
Read more »
