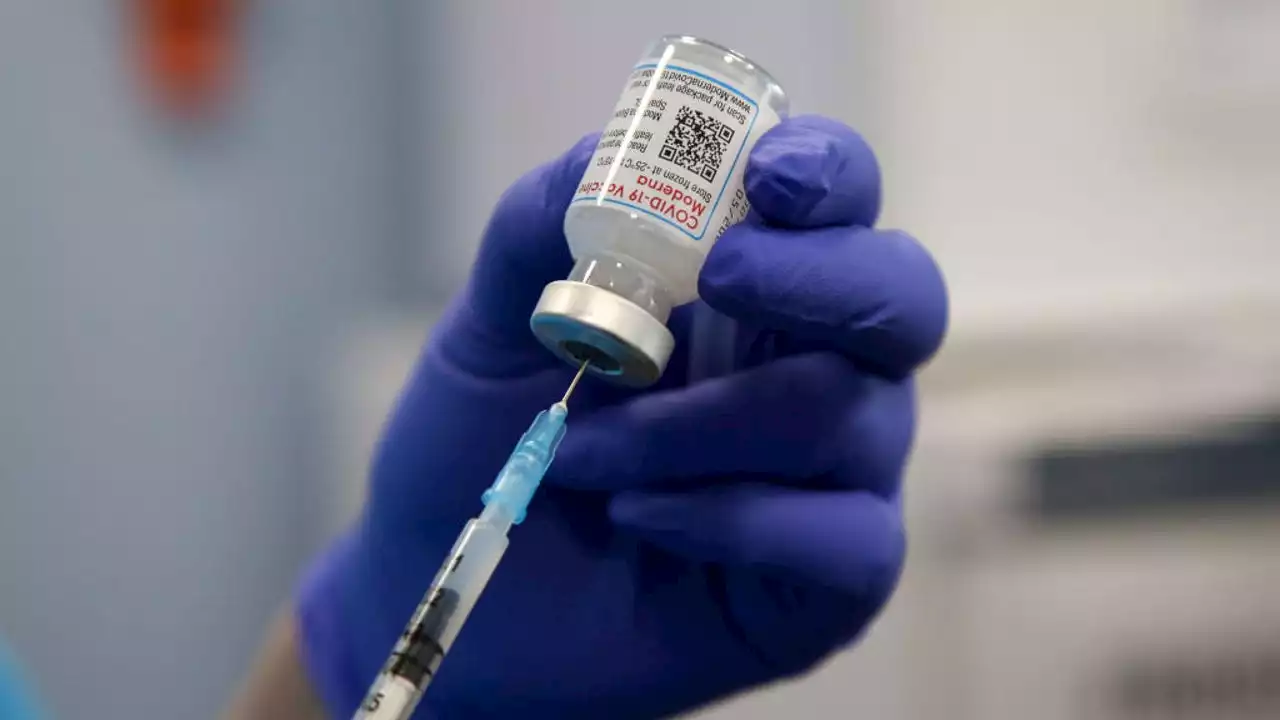
Moderna is asking U.S. regulators to approve its low-dose COVID-19 vaccine for children younger than 6, potentially opening shots for millions of tots by summer.
Moderna has asked the FDA for authorization on its COVID-19 vaccine to be administered in children under 6 years old. Moderna plans to provide two low-dose shots for babies, toddlers and preschoolers.for children younger than 6, a long-awaited move toward potentially opening shots for millions of tots by summer.
Moderna's vaccine isn't the only one in the race. Pfizer is soon expected to announce if three of its even smaller-dose shots work for the littlest kids, months after the disappointing discovery that two doses weren’t quite strong enough. "It’s critically important that we have the proper evaluation so that parents will have trust in any vaccines that we authorize," Marks told a Senate committee.The White House announced Vice President Kamala Harris has tested positive for COVID-19. In a statement, officials say Harris has not been in close contact to the President or First Lady due to their recent travel schedules.
"I will feel such a sense of relief when I know my boys are vaccinated and that the risk of them getting a serious infection is so low," she said.Some parents even have urged the government to let families choose shots before all the evidence is in.
Canada Latest News, Canada Headlines
Similar News:You can also read news stories similar to this one that we have collected from other news sources.
 Moderna Asks FDA To Approve COVID-19 Vaccine For Kids Under 6Moderna is also asking for approval of its other two vaccines for kids under 18.
Moderna Asks FDA To Approve COVID-19 Vaccine For Kids Under 6Moderna is also asking for approval of its other two vaccines for kids under 18.
Read more »
 Moderna asks FDA for authorization of its COVID-19 vaccine for children under 6The submission marks a hopeful development in the long journey for parents who are desperate to get their young kids vaccinated.
Moderna asks FDA for authorization of its COVID-19 vaccine for children under 6The submission marks a hopeful development in the long journey for parents who are desperate to get their young kids vaccinated.
Read more »
 Moderna Asks FDA to Approve Its COVID-19 Shot for Kids Under 6Moderna on Thursday asked U.S. regulators to authorize low doses of its COVID-19 vaccine for children younger than 6, a long-awaited move toward potentially opening shots for millions of tots by summer.
Moderna Asks FDA to Approve Its COVID-19 Shot for Kids Under 6Moderna on Thursday asked U.S. regulators to authorize low doses of its COVID-19 vaccine for children younger than 6, a long-awaited move toward potentially opening shots for millions of tots by summer.
Read more »
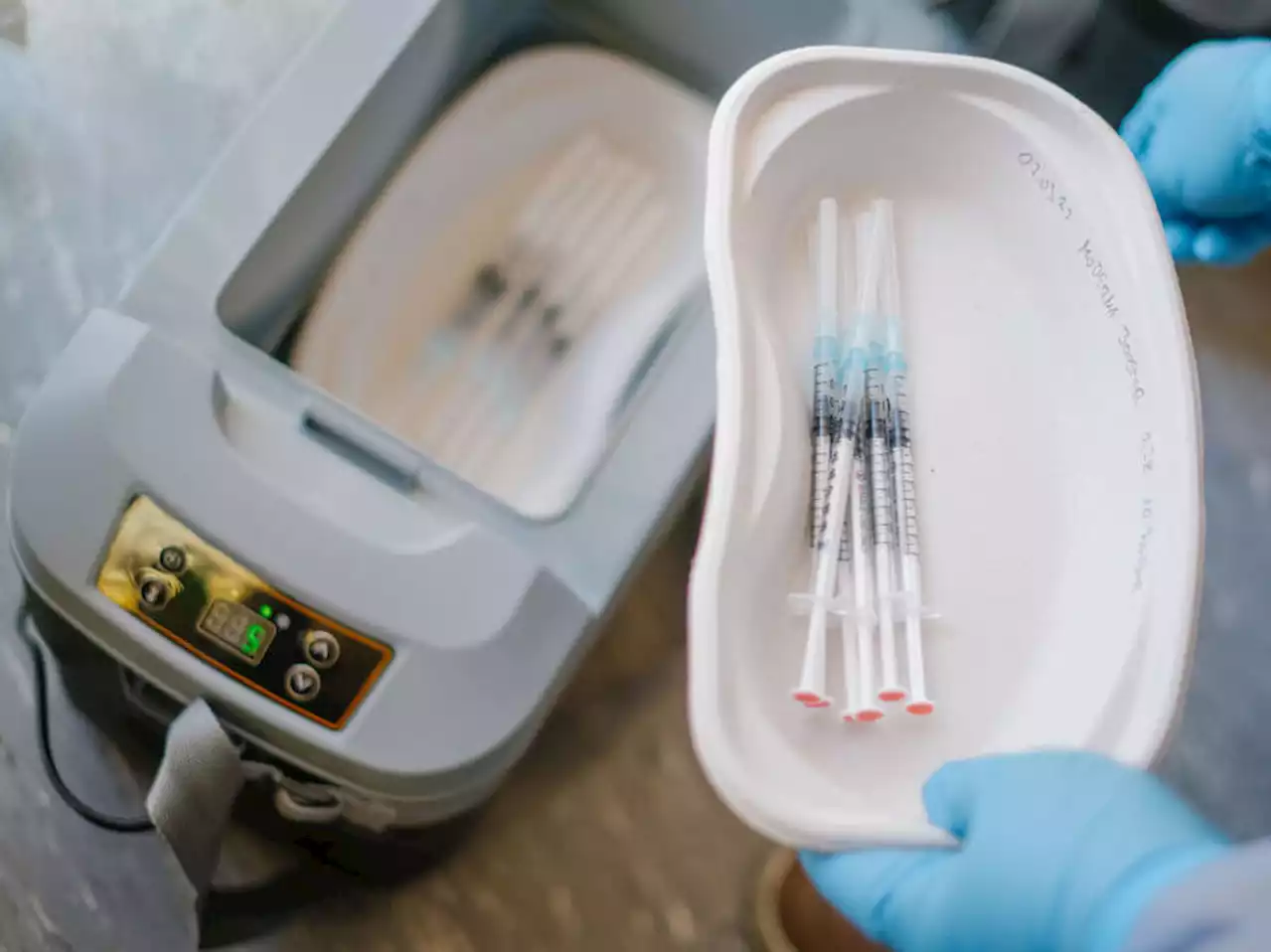 Moderna asks FDA to authorize first COVID-19 vaccine for very young childrenThe company says a low-dose version of its vaccine triggers an immune response in children ages 6 months to less than 6 years equivalent to what has protected older children and adults.
Moderna asks FDA to authorize first COVID-19 vaccine for very young childrenThe company says a low-dose version of its vaccine triggers an immune response in children ages 6 months to less than 6 years equivalent to what has protected older children and adults.
Read more »
 Moderna Asks FDA to Clear Its Covid-19 Vaccine for Young ChildrenModerna asked the FDA to authorize its Covid-19 vaccine for children 6 months to 5 years. If regulators agree, vaccinations could begin by summer.
Moderna Asks FDA to Clear Its Covid-19 Vaccine for Young ChildrenModerna asked the FDA to authorize its Covid-19 vaccine for children 6 months to 5 years. If regulators agree, vaccinations could begin by summer.
Read more »
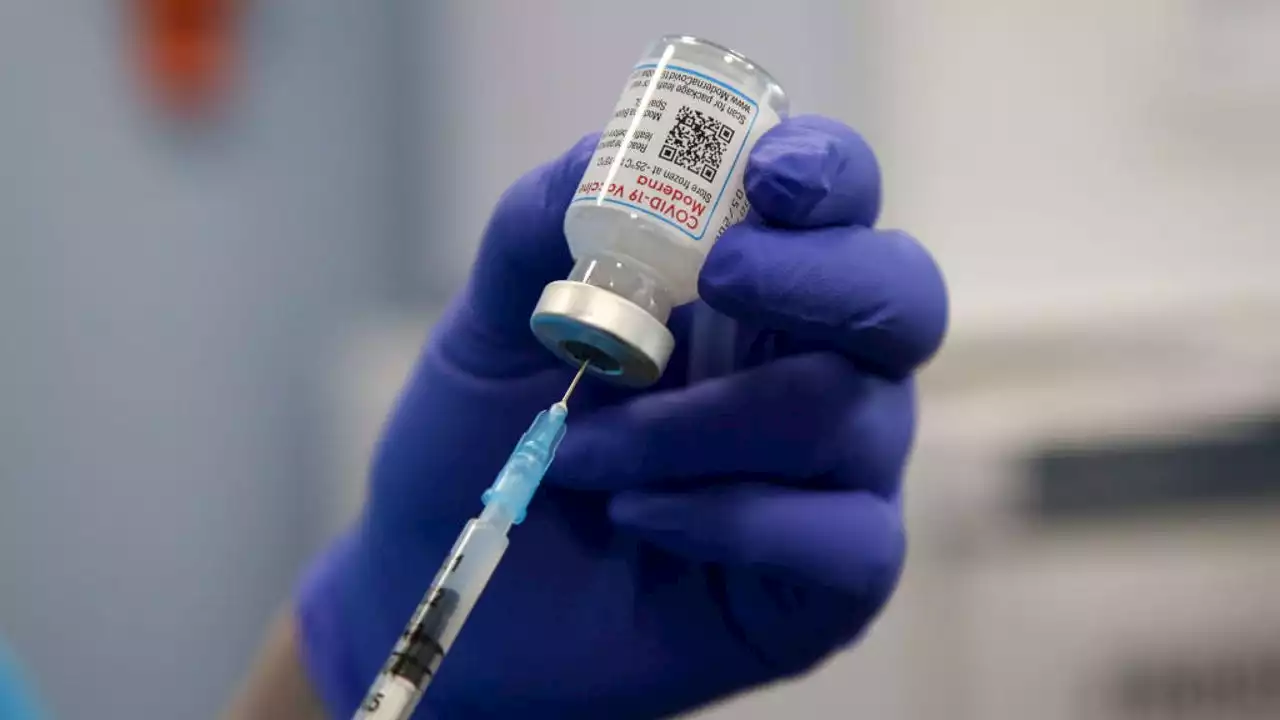 Moderna asks FDA to approve COVID-19 vaccine for kids under 6Moderna is asking U.S. regulators to approve its low-dose COVID-19 vaccine for children younger than 6, potentially opening shots for millions of tots by summer.
Moderna asks FDA to approve COVID-19 vaccine for kids under 6Moderna is asking U.S. regulators to approve its low-dose COVID-19 vaccine for children younger than 6, potentially opening shots for millions of tots by summer.
Read more »