JUST IN: Moderna asks FDA for authorization of its COVID-19 vaccine for children under 6.
hasn’t finished gathering its data yet, it's not yet clear which shot will be more effective.
But Burton defended the vaccine’s efficacy against infection, arguing that omicron led to more breakthrough infections, but that the shot produced an antibody response that was even stronger in the young kids than it was in the 18-24-year-olds. "We're working on a variant-specific booster, we released some data a couple of weeks ago. We're still working on even another booster candidate that will cover omicron, as well as the original virus. And if these little kids do need an additional booster, I think that's the one that we would likely offer them in the fall or winter,” Burton said.But Burton said he was confident in the two-dose regimen Moderna is putting before the FDA.
For parents, the authorization of vaccines for the youngest population in the country has been a stressful, arduous wait.
Canada Latest News, Canada Headlines
Similar News:You can also read news stories similar to this one that we have collected from other news sources.
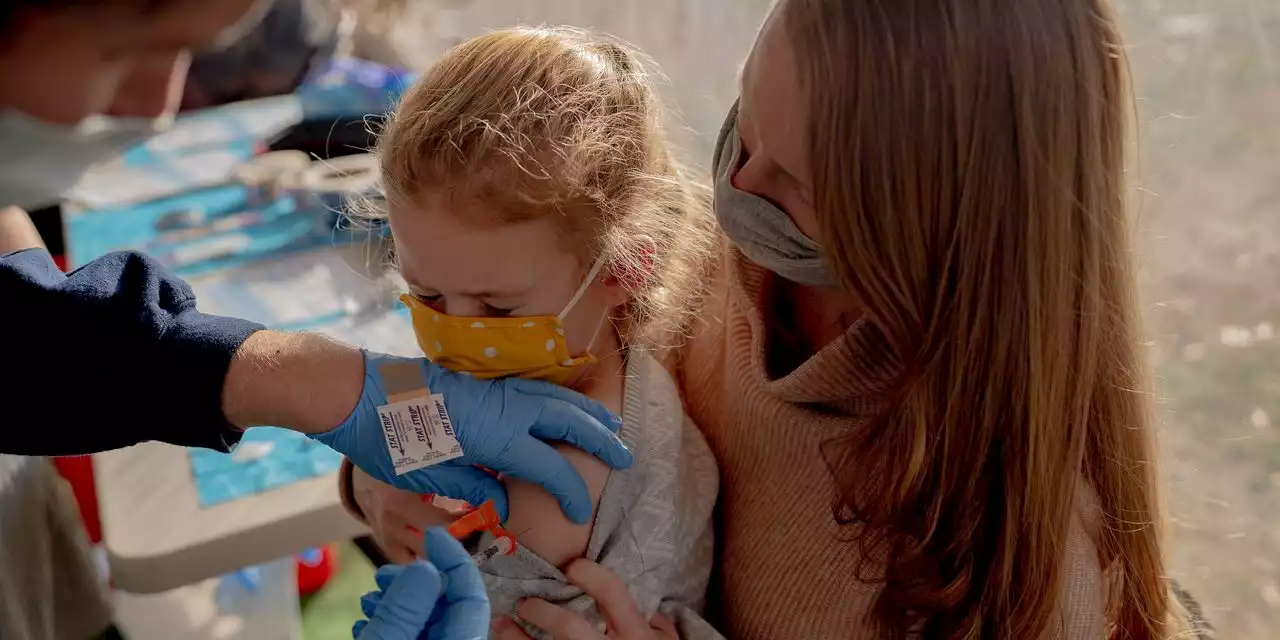 Pfizer Asks FDA to Authorize Covid-19 Booster for 5- to 11-Year-OldsPfizer Inc. and partner BioNTech SE said earlier this month that a third shot safely generated a strong immune response in the youngsters.
Pfizer Asks FDA to Authorize Covid-19 Booster for 5- to 11-Year-OldsPfizer Inc. and partner BioNTech SE said earlier this month that a third shot safely generated a strong immune response in the youngsters.
Read more »
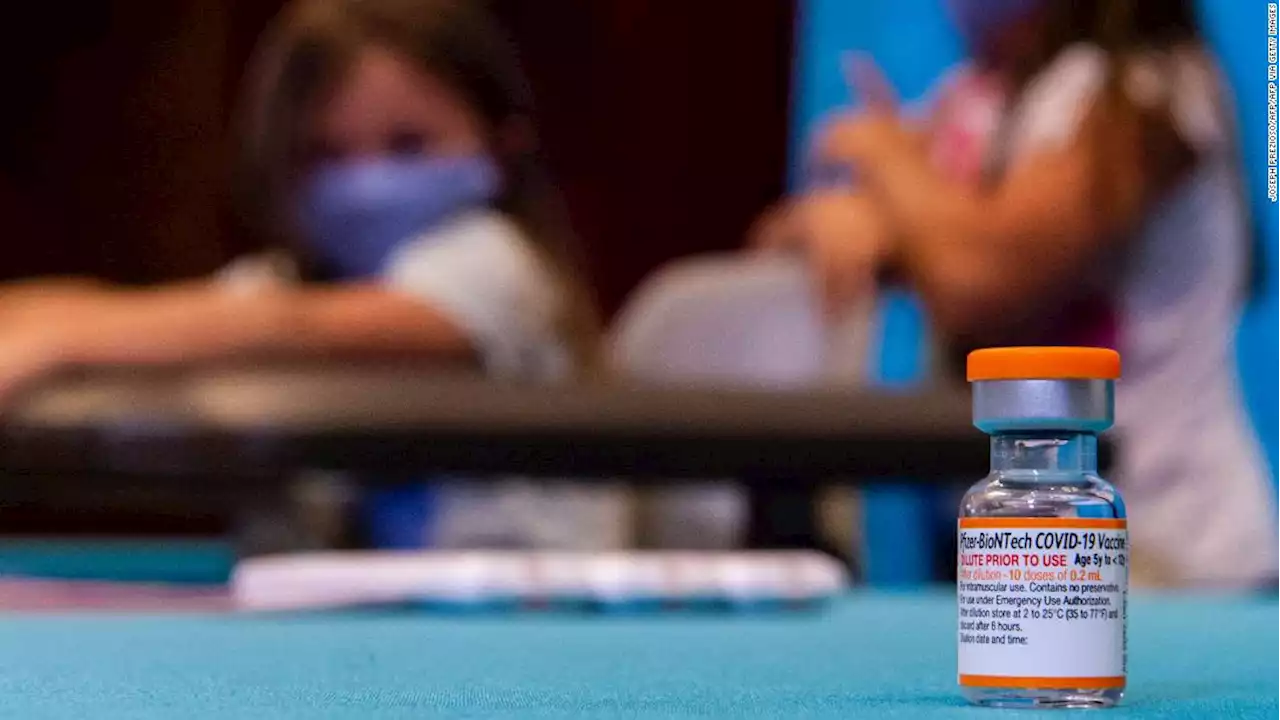 Pfizer requests FDA authorization for Covid-19 booster for kids 5 through 11Pfizer and BioNTech said Tuesday that they have submitted an application to the US Food and Drug Administration for emergency use authorization of a booster dose of Covid-19 vaccine for children 5 through 11.
Pfizer requests FDA authorization for Covid-19 booster for kids 5 through 11Pfizer and BioNTech said Tuesday that they have submitted an application to the US Food and Drug Administration for emergency use authorization of a booster dose of Covid-19 vaccine for children 5 through 11.
Read more »
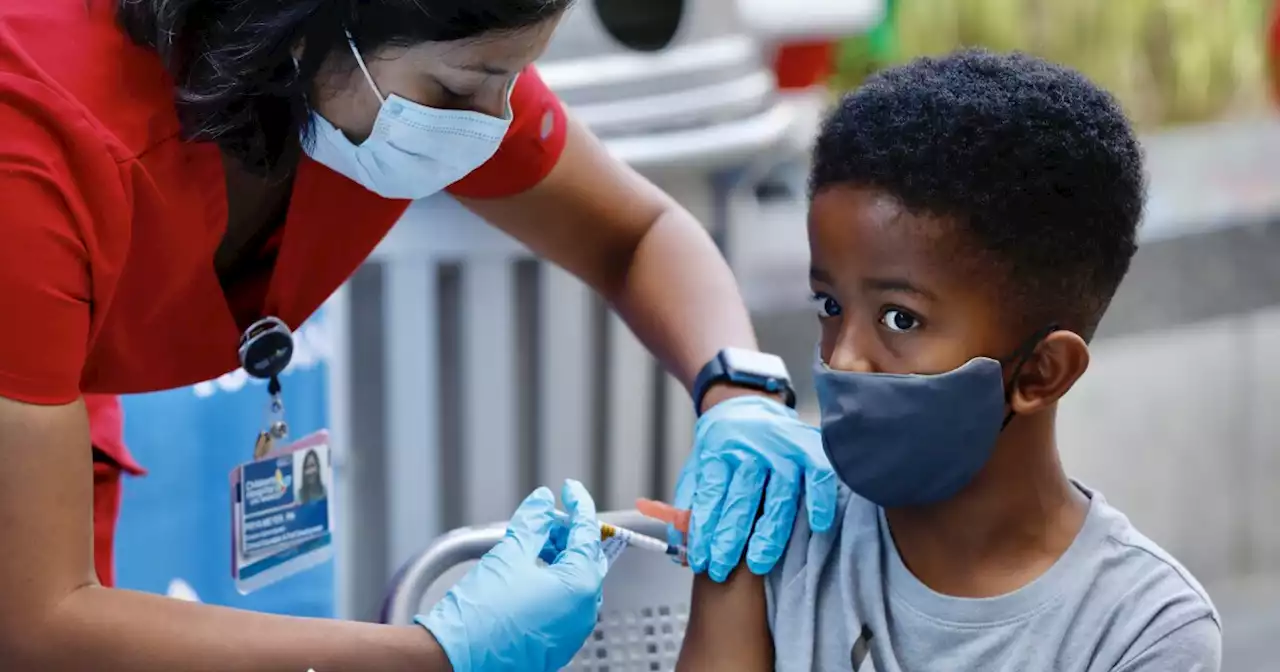 Pfizer asks FDA to clear COVID-19 booster shot for children ages 5 to 11Pfizer asked U.S. regulators for emergency-use authorization of a booster shot of its COVID-19 vaccine in children ages 5 to 11.
Pfizer asks FDA to clear COVID-19 booster shot for children ages 5 to 11Pfizer asked U.S. regulators for emergency-use authorization of a booster shot of its COVID-19 vaccine in children ages 5 to 11.
Read more »
 Moderna to ask FDA to authorize Covid-19 vaccine for young kidsThe request comes a month after the drug company signaled its two-dose regimen generated immune protection in the youngest children comparable to young adults.
Moderna to ask FDA to authorize Covid-19 vaccine for young kidsThe request comes a month after the drug company signaled its two-dose regimen generated immune protection in the youngest children comparable to young adults.
Read more »
 Moderna Asks FDA to Approve Its COVID-19 Shot for Kids Under 6Moderna on Thursday asked U.S. regulators to authorize low doses of its COVID-19 vaccine for children younger than 6, a long-awaited move toward potentially opening shots for millions of tots by summer.
Moderna Asks FDA to Approve Its COVID-19 Shot for Kids Under 6Moderna on Thursday asked U.S. regulators to authorize low doses of its COVID-19 vaccine for children younger than 6, a long-awaited move toward potentially opening shots for millions of tots by summer.
Read more »
 Moderna asks FDA to authorize first COVID-19 vaccine for very young childrenThe company says a low-dose version of its vaccine triggers an immune response in children ages 6 months to less than 6 years equivalent to what has protected older children and adults.
Moderna asks FDA to authorize first COVID-19 vaccine for very young childrenThe company says a low-dose version of its vaccine triggers an immune response in children ages 6 months to less than 6 years equivalent to what has protected older children and adults.
Read more »
