The results suggest that GSK’s shot could help protect a wider population from RSV, which causes thousands of hospitalizations and deaths each year.
A vaccine from GlaxoSmithKline showed the potential to protect adults ages 50 to 59 from respiratory syncytial virus in a late-stage clinical trial.
The shot, known as Arexvy, is approved in the U.S., Europe, Japan and other countries for adults ages 60 and older., could help protect a wider population from RSV, a disease that causes thousands of hospitalizations and deaths among older Americans each year. Currently, Arexvy isA single dose of the British drugmaker's shot elicited an immune response in adults ages 50 to 59 who are at an increased risk of catching RSV due to certain underlying medical conditions.
GSK said it plans to present final results from the trial at an upcoming medical conference and submit them for publication in a peer-reviewed journal. The company added that it is"on track" to become the first company to submit data on this age group to the Food and Drug Administration and other regulators and expects a decision on a potential label expansion in 2024.
U.S. health officials are banking on the shots from Pfizer and GSK shots to help the country combat this year's RSV season. RSV and other respiratory viruses such as the flu are already starting to circulate, but so far at lower rates than this time last year, the CDC said last week.
Canada Latest News, Canada Headlines
Similar News:You can also read news stories similar to this one that we have collected from other news sources.
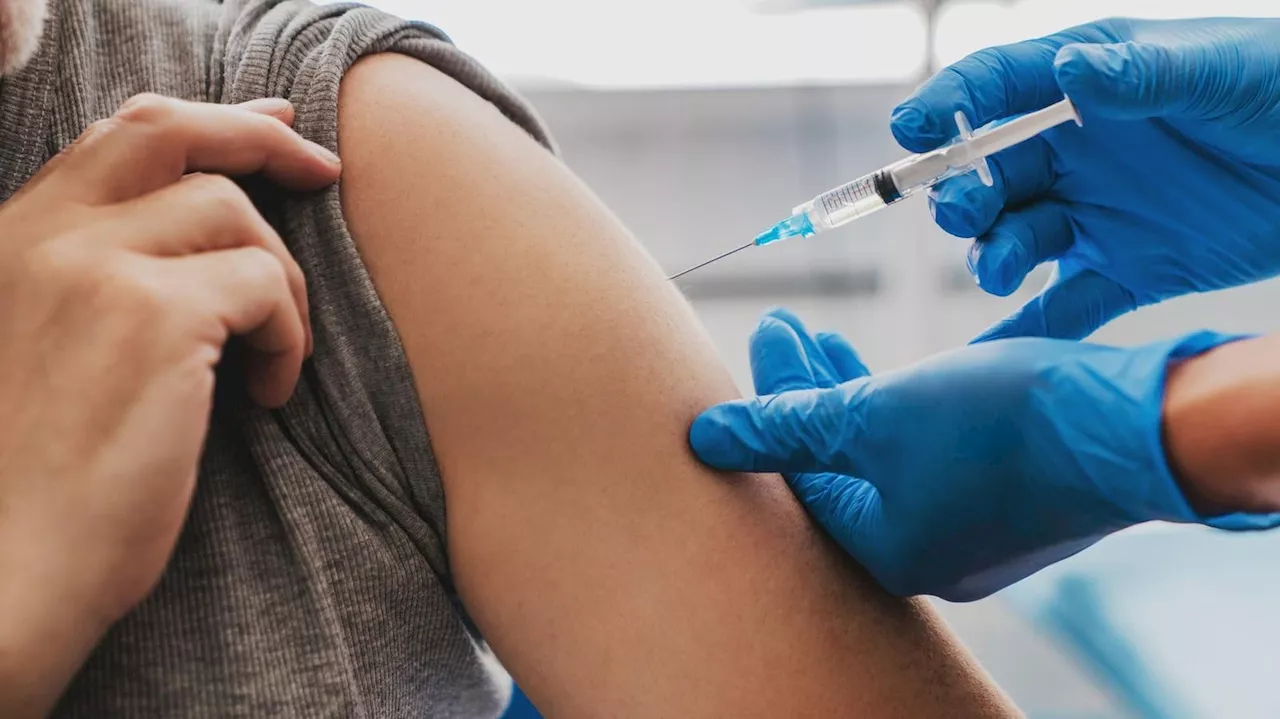 RSV Vaccine Shows Hopeful Early Results In 50 To 59-Year-Olds, GSK SaysI am a senior reporter for the Forbes breaking news team, covering health and science from the London office. Previously I worked as a reporter for a trade publication covering big data and law and as a freelance journalist and policy analyst covering science, tech and health.
RSV Vaccine Shows Hopeful Early Results In 50 To 59-Year-Olds, GSK SaysI am a senior reporter for the Forbes breaking news team, covering health and science from the London office. Previously I worked as a reporter for a trade publication covering big data and law and as a freelance journalist and policy analyst covering science, tech and health.
Read more »
 RSV vaccine from GSK shows potential to protect adults 50 to 59The results suggest that GSK’s shot could help protect a wider population from RSV, which causes thousands of hospitalizations and deaths each year.
RSV vaccine from GSK shows potential to protect adults 50 to 59The results suggest that GSK’s shot could help protect a wider population from RSV, which causes thousands of hospitalizations and deaths each year.
Read more »
 Doctors asked to prioritize which infants should get new RSV shotDoctors are being asked to preserve units of the new RSV shot, as demand is outweighing supply.
Doctors asked to prioritize which infants should get new RSV shotDoctors are being asked to preserve units of the new RSV shot, as demand is outweighing supply.
Read more »
 CDC Issues RSV Vaccination Alert Because of Drug ShortageA limited supply of a drug to protect infants from the respiratory disease RSV has led the CDC to advise pediatricians to use certain doses of nirsevimab, a monoclonal antibody sold by the brand name Beyfortus, for infants with the greatest danger of developing severe respiratory syncytial virus.
CDC Issues RSV Vaccination Alert Because of Drug ShortageA limited supply of a drug to protect infants from the respiratory disease RSV has led the CDC to advise pediatricians to use certain doses of nirsevimab, a monoclonal antibody sold by the brand name Beyfortus, for infants with the greatest danger of developing severe respiratory syncytial virus.
Read more »
 A new RSV shot for infants is in short supplyEdwaard Liang, one of the first and only Chinese-American artistic directors in the country, accepted an offer as the artistic director for the Washington Ballet.
A new RSV shot for infants is in short supplyEdwaard Liang, one of the first and only Chinese-American artistic directors in the country, accepted an offer as the artistic director for the Washington Ballet.
Read more »
