The Food and Drug Administration approved the once-a-day pill from Astellas Pharma to treat moderate-to-severe symptoms, which can include sweating, flushing and chills.
Astellas’ drug, Veozah, uses a new approach, targeting brain connections that help control body temperature. The FDA said the medication will provide “an additional safe and effective treatment option for women,” in a statement.
More than 80% of women experience hot flashes during menopause, the FDA noted, as the body gradually produces lower levels of reproductive hormones between the ages of 45 and 55. The most common treatment consists of hormonal pills aimed at boosting levels of estrogen and progestin. But the treatment isn’t appropriate for some women, including those with a history of stroke, blood clots, heart attack and other health conditions. Large studies have found that the hormones can increase the chances of those problems reoccurring, although the risks vary based on a number of individual factors.
The new pill is not a hormone. It carries an FDA warning about potential liver damage. Women will need to be screened for liver damage or infection before getting a prescription, then get a blood test every three months for nine months to monitor for safety problems, according to the FDA label. Astellas said the drug will cost $550 for a one-month supply. That’s the price before insurance coverage and other discounts typically negotiated by insurers and pharmacy benefit managers.The Associated Press Health and Science Department receives support from the Howard Hughes Medical Institute’s Science and Educational Media Group. The AP is solely responsible for all content.
Canada Latest News, Canada Headlines
Similar News:You can also read news stories similar to this one that we have collected from other news sources.
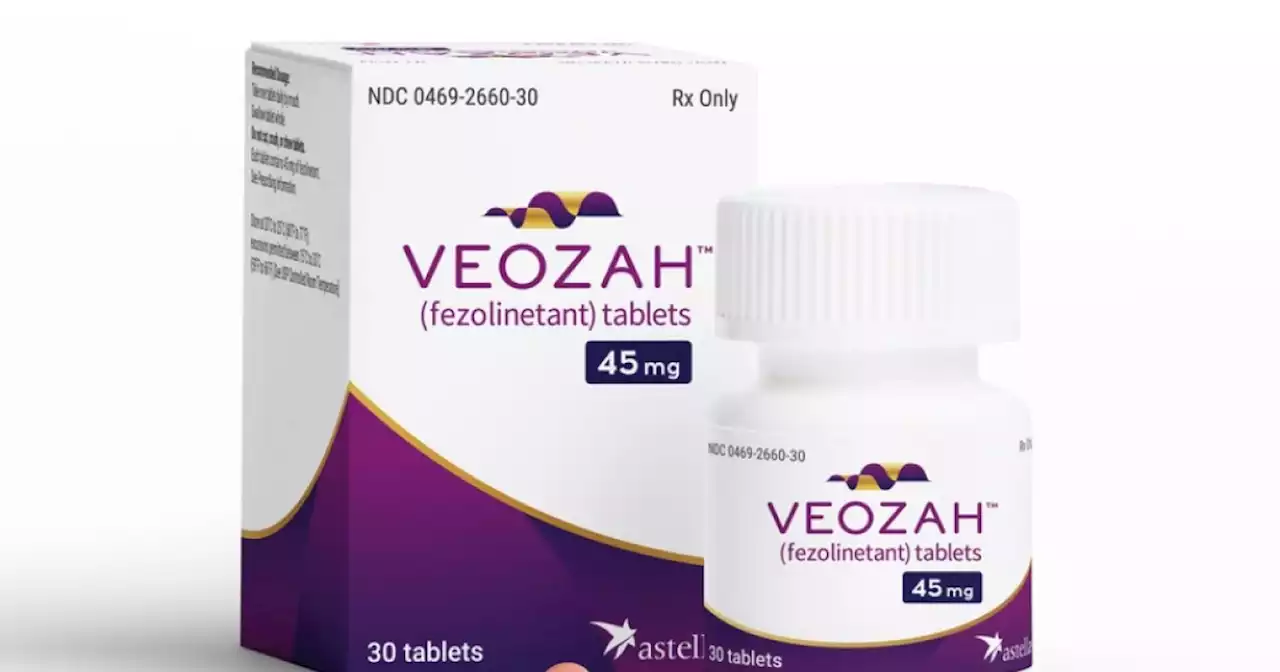 FDA approves new type of drug to treat menopausal hot flashesThe FDA has approved a once-a-day pill that neurologically targets the body's temperature control center, which can calm menopausal hot flashes.
FDA approves new type of drug to treat menopausal hot flashesThe FDA has approved a once-a-day pill that neurologically targets the body's temperature control center, which can calm menopausal hot flashes.
Read more »
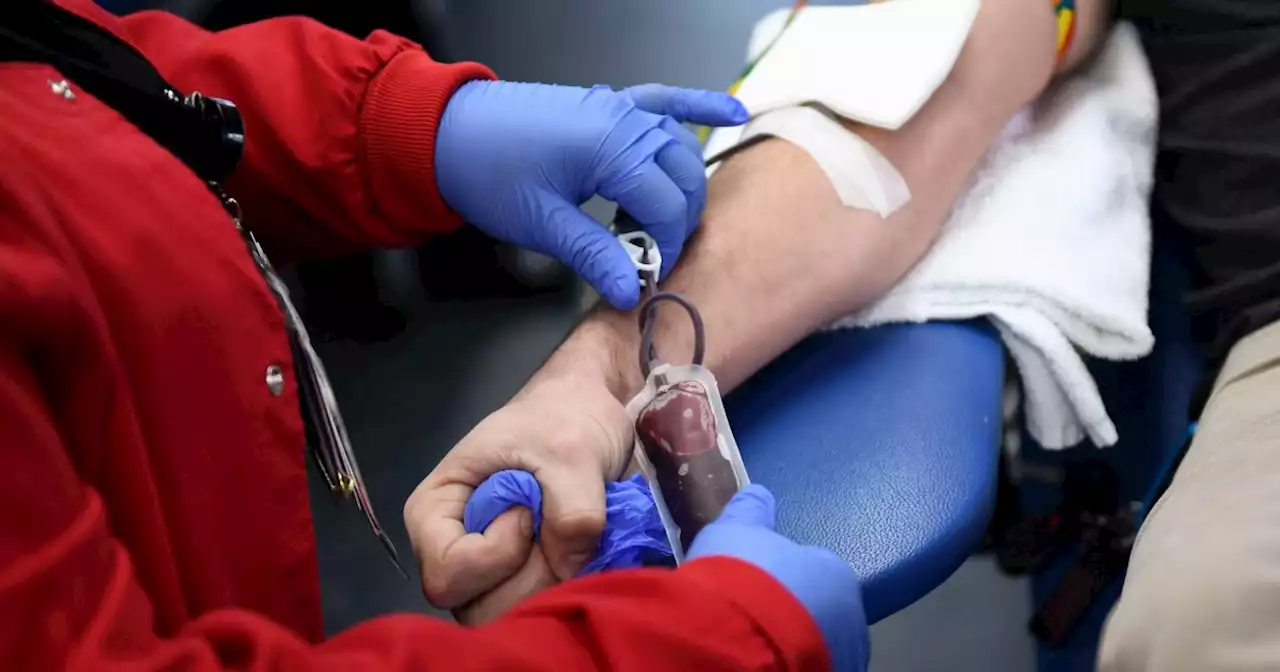 More gay and bisexual men can donate blood under new FDA rulesMost gay and bisexual men who are in a monogamous relationship with another man will no longer need to abstain from sex to donate blood.
More gay and bisexual men can donate blood under new FDA rulesMost gay and bisexual men who are in a monogamous relationship with another man will no longer need to abstain from sex to donate blood.
Read more »
 FDA finalizes new rules on blood donation for gay and bisexual menThe step paves the way for more blood donors and represents another step in ending a discriminatory and outdated policy.
FDA finalizes new rules on blood donation for gay and bisexual menThe step paves the way for more blood donors and represents another step in ending a discriminatory and outdated policy.
Read more »
 New FDA Rule Allows More Gay and Bisexual Men to Donate BloodGay and bisexual men were previously subject to a lifetime ban on blood donations, which was widely regarded as discriminatory.
New FDA Rule Allows More Gay and Bisexual Men to Donate BloodGay and bisexual men were previously subject to a lifetime ban on blood donations, which was widely regarded as discriminatory.
Read more »
 FDA OKs New Drug for Fabry DiseasePegunigalsidase alfa (Elfabrio) is an intravenously administered enzyme replacement therapy to treat adults with confirmed Fabry disease.
FDA OKs New Drug for Fabry DiseasePegunigalsidase alfa (Elfabrio) is an intravenously administered enzyme replacement therapy to treat adults with confirmed Fabry disease.
Read more »
 New FDA rules allow more gay, bisexual men to donate bloodGay rights groups have long opposed blanket restrictions on who can give blood, saying they discriminate. Medical societies including the American Medical Association have also said such exclusions…
New FDA rules allow more gay, bisexual men to donate bloodGay rights groups have long opposed blanket restrictions on who can give blood, saying they discriminate. Medical societies including the American Medical Association have also said such exclusions…
Read more »
