An experimental HIV vaccine has been found to induce broadly neutralizing antibodies among a small group of volunteers in a Phase 1 study.
The clinical trial results, published Thursday on World AIDS Day in the journal Science, establish “clinical proof of concept” in support of developing boosting regimens to induce immune responses against HIV infection, for which there is no cure and which can cause acquired immunodeficiency syndrome, known as AIDS.
What is unique about this HIV vaccine candidate is that it was engineered to directly target the production of broadly neutralizing antibodies, said Dr. Timothy Schacker, vice dean for research and program director in HIV medicine at the University of Minnesota Medical School, who was not involved in the research. “In HIV, when we’ve designed and tested vaccines in the past, they didn’t for whatever reason induce these broadly neutralizing antibodies,” he said.
Canada Latest News, Canada Headlines
Similar News:You can also read news stories similar to this one that we have collected from other news sources.
 First on CNN: US considers dramatically expanding training of Ukrainian forces, US officials say | CNN PoliticsThe Biden administration is considering a dramatic expansion in the training the US military provides to Ukrainian forces, including instructing as many as 2,500 Ukrainian soldiers a month at a US base in Germany, according to multiple US officials.
First on CNN: US considers dramatically expanding training of Ukrainian forces, US officials say | CNN PoliticsThe Biden administration is considering a dramatic expansion in the training the US military provides to Ukrainian forces, including instructing as many as 2,500 Ukrainian soldiers a month at a US base in Germany, according to multiple US officials.
Read more »
 Safety and antiviral activity of triple combination broadly neutralizing monoclonal antibody therapy against HIV-1: a phase 1 clinical trial - Nature MedicineA combination of three monoclonal antibodies transiently reduced viremia in people living with HIV-1 and not on antiretroviral therapy, but it did not prevent viral rebound. Further studies are needed to determine if this approach can be optimized.
Safety and antiviral activity of triple combination broadly neutralizing monoclonal antibody therapy against HIV-1: a phase 1 clinical trial - Nature MedicineA combination of three monoclonal antibodies transiently reduced viremia in people living with HIV-1 and not on antiretroviral therapy, but it did not prevent viral rebound. Further studies are needed to determine if this approach can be optimized.
Read more »
 Safety and virologic impact of the IL-15 superagonist N-803 in people living with HIV: a phase 1 trial - Nature MedicineAn exploratory analysis of IL-15 superagonist treatment in people living with HIV and taking antiretroviral therapy shows increased T cell activation and viral transcription alongside a sustained reduction in inducible HIV provirus from blood cells.
Safety and virologic impact of the IL-15 superagonist N-803 in people living with HIV: a phase 1 trial - Nature MedicineAn exploratory analysis of IL-15 superagonist treatment in people living with HIV and taking antiretroviral therapy shows increased T cell activation and viral transcription alongside a sustained reduction in inducible HIV provirus from blood cells.
Read more »
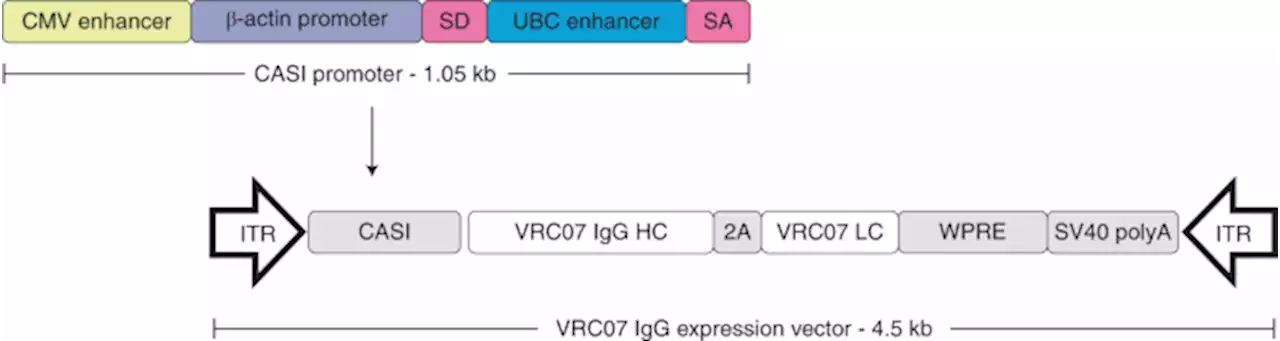 Safety and tolerability of AAV8 delivery of a broadly neutralizing antibody in adults living with HIV: a phase 1, dose-escalation trial - Nature MedicineResults from a phase 1 clinical trial show that a broadly neutralizing, HIV-specific antibody is durably expressed in vivo from a viral vector, highlighting an alternate approach for the delivery of antibodies in humans NIAIDNews HIV WorldAIDSDay
Safety and tolerability of AAV8 delivery of a broadly neutralizing antibody in adults living with HIV: a phase 1, dose-escalation trial - Nature MedicineResults from a phase 1 clinical trial show that a broadly neutralizing, HIV-specific antibody is durably expressed in vivo from a viral vector, highlighting an alternate approach for the delivery of antibodies in humans NIAIDNews HIV WorldAIDSDay
Read more »