An FDA panel voted unanimously to support Eli Lilly's Alzheimer's drug for use in adults 60-85 years old.
The monoclonal antibody drug, delivered through an IV monthly for up to 18 months, would be the second Alzheimer's drug on the market if the FDA moves ahead to approve. The FDA 's decision does not have to align with the panel's vote.
Leqembi has struggled to take off due to burdensome Medicare rules for use — including expensive brain scans — as well asEisai and Biogen have also asked the FDA to approve an injectable version of the drug, which would make it easier for patients to access. Mamie Laverock's Family Shares Photo of Actress' Hand from Her Hospital Bed Following Five-Story Fall7 Democrats who could replace Biden if he drops his 2024 reelection bid
FDA Eisai Alzheimer's Drug Monoclonal Antibody
Canada Latest News, Canada Headlines
Similar News:You can also read news stories similar to this one that we have collected from other news sources.
 Lilly Alzheimer’s Drug Seen Effective by FDA Advisory PanelEli Lilly & Co.’s treatment for Alzheimer’s disease works in at least some patients, according to a US advisory panel.
Lilly Alzheimer’s Drug Seen Effective by FDA Advisory PanelEli Lilly & Co.’s treatment for Alzheimer’s disease works in at least some patients, according to a US advisory panel.
Read more »
 FDA Staff Proposes Narrow Approval for Lilly’s Alzheimer’s DrugUS drug regulatory staffers are considering a more targeted approval for Eli Lilly & Co.’s experimental Alzheimer’s disease treatment than the company wants, potentially limiting the market for a would-be blockbuster.
FDA Staff Proposes Narrow Approval for Lilly’s Alzheimer’s DrugUS drug regulatory staffers are considering a more targeted approval for Eli Lilly & Co.’s experimental Alzheimer’s disease treatment than the company wants, potentially limiting the market for a would-be blockbuster.
Read more »
 Eli Lilly’s Obesity Drug Crunch Shows Signs of Easing in JapanEli Lilly & Co.’s hefty investment in expanding production capacity for its popular weight-loss and diabetes drugs is starting to pay off in Japan, allowing the company to lift shipment controls it imposed in the country last year.
Eli Lilly’s Obesity Drug Crunch Shows Signs of Easing in JapanEli Lilly & Co.’s hefty investment in expanding production capacity for its popular weight-loss and diabetes drugs is starting to pay off in Japan, allowing the company to lift shipment controls it imposed in the country last year.
Read more »
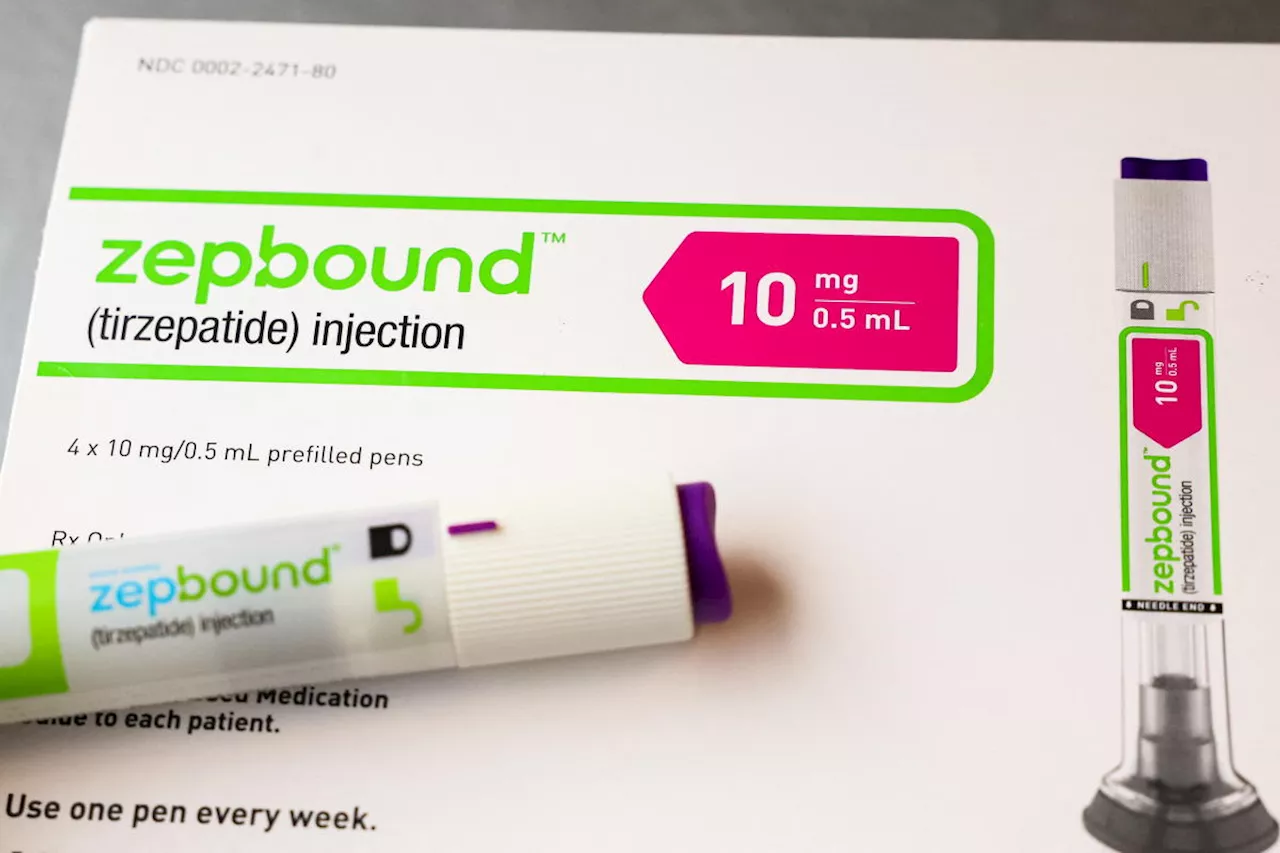 Eli Lilly exec on weight-loss drugs: 'It's not just a flash in the pan'Eli Lilly may be riding high on its diabetes and weight-loss treatments Mounjaro and Zepbound — which Wall Street estimates could net the company $13.5...
Eli Lilly exec on weight-loss drugs: 'It's not just a flash in the pan'Eli Lilly may be riding high on its diabetes and weight-loss treatments Mounjaro and Zepbound — which Wall Street estimates could net the company $13.5...
Read more »
 Eli Lilly reaches settlement with spa selling Mounjaro, Zepbound knockoffsExplore stories from Atlantic Canada.
Eli Lilly reaches settlement with spa selling Mounjaro, Zepbound knockoffsExplore stories from Atlantic Canada.
Read more »
 China Clears Eli Lilly’s Weight-Loss Drug, Only for Diabetes NowChina gave the greenlight to Eli Lilly & Co.’s blockbuster drug tirzepatide for use in treating diabetes, a move that will intensify the drugmaker’s rivalry with Novo Nordisk A/S over weight-loss drugs in the world’s second-largest market.
China Clears Eli Lilly’s Weight-Loss Drug, Only for Diabetes NowChina gave the greenlight to Eli Lilly & Co.’s blockbuster drug tirzepatide for use in treating diabetes, a move that will intensify the drugmaker’s rivalry with Novo Nordisk A/S over weight-loss drugs in the world’s second-largest market.
Read more »
