🚨FDA has confirmed a nationwide shortage of the immediate release formulation of amphetamine mixed salts, Adderall, which is approved for treating ADHD and narcolepsy. MedTwitter
The FDA announcement follows weeks of reports of a shortage of the drug by pharmacy chains and Adderall users,The agency said it is in"frequent" contact with all manufacturers of Adderall — and reported that one of those companies, Teva, is experiencing ongoing intermittent manufacturing delays.
Other manufacturers continue to produce amphetamine mixed salts, but there is not enough supply to continue to meet US market demand through those producers, the FDA noted. "Until supply is restored, there are alternative therapies including the extended-release version of amphetamine mixed salts available to health care professionals and their patients for amphetamine mixed salts' approved indications," the agency said.
Patients should work with their healthcare provider to determine their best treatment option, it added. The organization is continuing to monitor the supply of Adderall and to help manufacturers resolve the shortage."We continue to use all the tools we have available to help keep supply available for patients and will provide public updates regarding the Adderall shortage," the FDA said.
Canada Latest News, Canada Headlines
Similar News:You can also read news stories similar to this one that we have collected from other news sources.
 There's Officially a Nationwide Adderall ShortageThe Food and Drug Administration has announced a shortage of the commonly prescribed ADHD drug. Doctors and patients are scrambling to find alternatives.
There's Officially a Nationwide Adderall ShortageThe Food and Drug Administration has announced a shortage of the commonly prescribed ADHD drug. Doctors and patients are scrambling to find alternatives.
Read more »
 FDA authorizes omicron-targeting COVID-19 booster for younger kidsThe Food and Drug Administration authorized the bivalent COVID-19 booster shot for younger children. Moderna's booster is authorized for children six years and older.
FDA authorizes omicron-targeting COVID-19 booster for younger kidsThe Food and Drug Administration authorized the bivalent COVID-19 booster shot for younger children. Moderna's booster is authorized for children six years and older.
Read more »
 FDA authorizes updated bivalent COVID-19 booster for children aged 5 and olderThe Food and Drug Administration authorized the updated bivalent COVID-19 booster for children ages 5 and older Monday.
FDA authorizes updated bivalent COVID-19 booster for children aged 5 and olderThe Food and Drug Administration authorized the updated bivalent COVID-19 booster for children ages 5 and older Monday.
Read more »
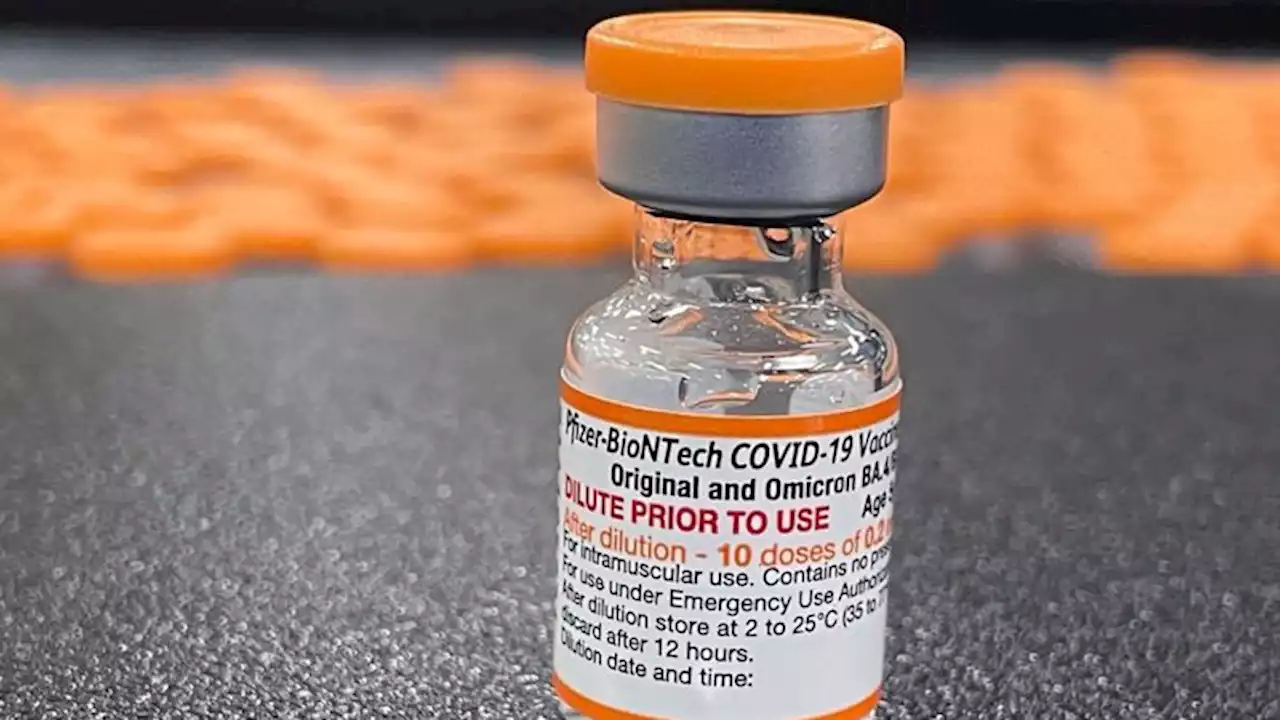 FDA authorizes updated Covid-19 booster shots for children as young as 5 | CNNFDA authorizes updated Covid-19 booster shots for children as young as 5. The boosters target the original coronavirus strain and Omicron subvariants.
FDA authorizes updated Covid-19 booster shots for children as young as 5 | CNNFDA authorizes updated Covid-19 booster shots for children as young as 5. The boosters target the original coronavirus strain and Omicron subvariants.
Read more »
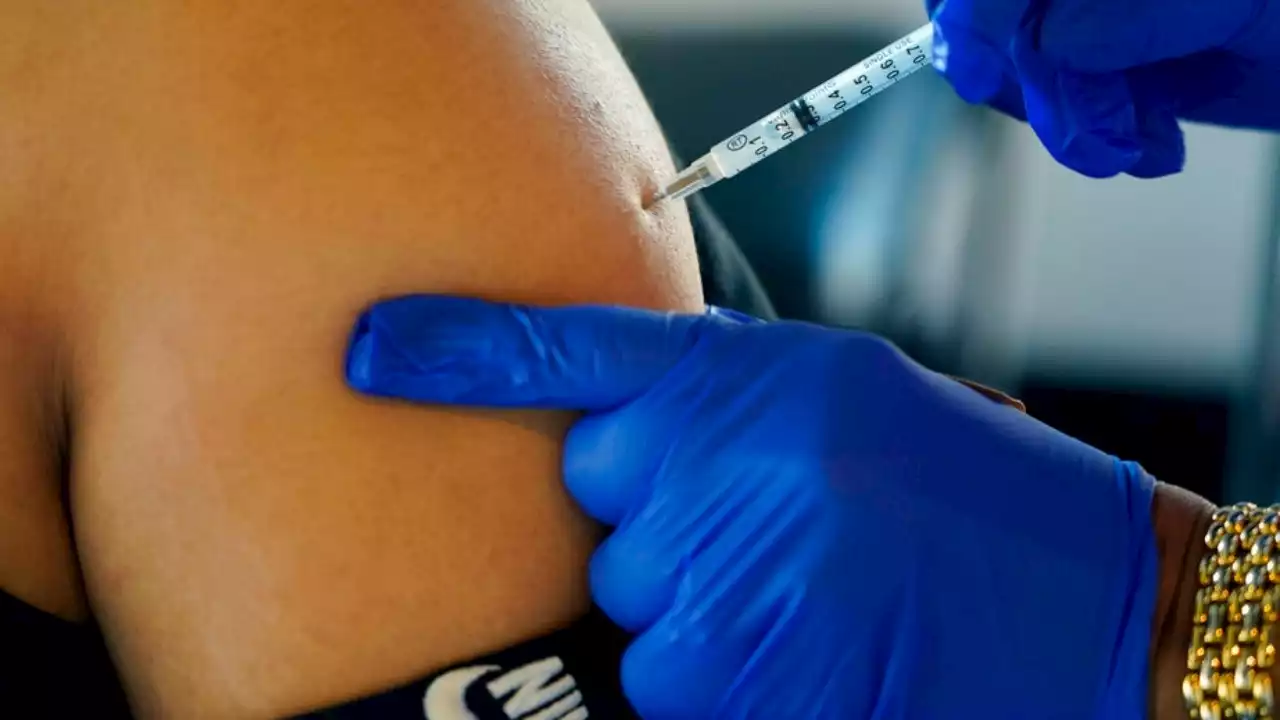 FDA authorizes bivalent COVID boosters for young childrenThe Food and Drug Administration announced Wednesday that it was amending is emergency use authorization of bivalent COVID vaccines for use in younger kids.
FDA authorizes bivalent COVID boosters for young childrenThe Food and Drug Administration announced Wednesday that it was amending is emergency use authorization of bivalent COVID vaccines for use in younger kids.
Read more »
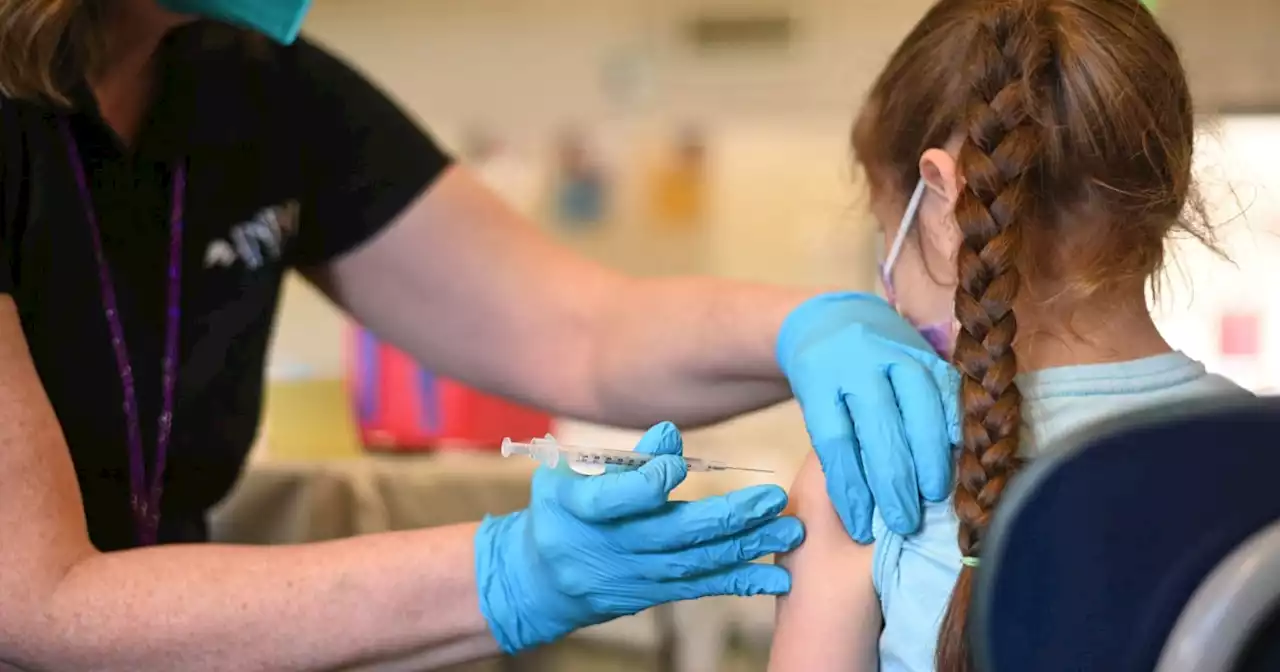 Children as young as 5 can now get omicron booster shots, CDC saysCDC Director Dr. Rochelle Walensky signed off on the updated Covid vaccines only hours after authorization from the Food and Drug Administration.
Children as young as 5 can now get omicron booster shots, CDC saysCDC Director Dr. Rochelle Walensky signed off on the updated Covid vaccines only hours after authorization from the Food and Drug Administration.
Read more »
