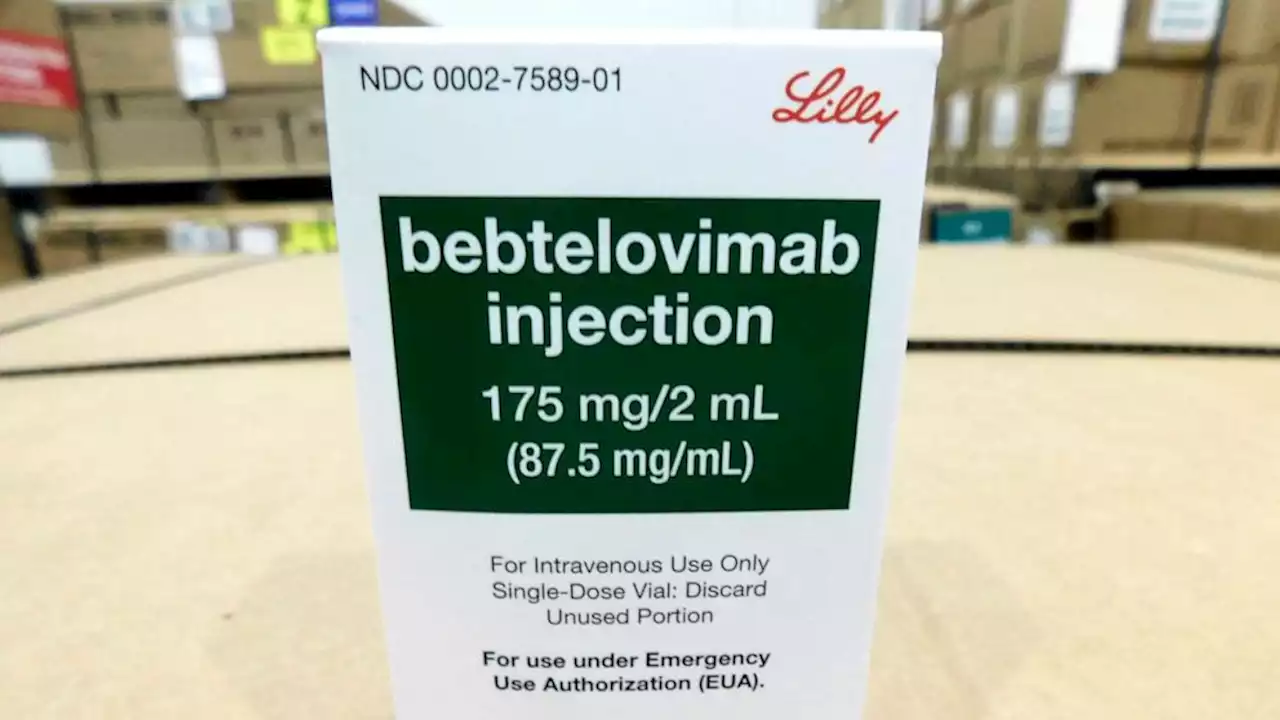
The FDA has authorized a new monoclonal antibody treatment for COVID-19, shown to hold up against the omicron variant and BA.2 subvariant.
On the eve of authorization, the Biden administration announced it had purchased 600,000 doses of bebtelovimab for at least $720 million. The plan is to get roughly 300,000 doses out this month, and another 300,000 in March. The contract also includes a future option for 500,000 more doses, if necessary.
Daniel Skovronsky, Eli Lilly's chief scientific and medical officer and president of their research labs, said in an interview with ABC News that the company began working on the therapy more than a year ago -- long before omicron emerged -- in hopes of being"future proof" for a range of variants and anything else that might come down the road.This image provided by Eli Lilly and Company shows the packaging for bebtelovimab.
Canada Latest News, Canada Headlines
Similar News:You can also read news stories similar to this one that we have collected from other news sources.
 US preps 10 million doses of COVID-19 vaccine for kids under 5, pending FDA authorizationCOVID-19 vaccines for kids under 5 have not yet been authorized, but the rollout plan is already in the works.
US preps 10 million doses of COVID-19 vaccine for kids under 5, pending FDA authorizationCOVID-19 vaccines for kids under 5 have not yet been authorized, but the rollout plan is already in the works.
Read more »
 Young kids’ COVID vaccines create approval dilemma for FDATwo doses of Pfizer's vaccine showed mixed results in trials, but the agency is considering approving it anyways in anticipation that a future third shot will provide children with strong immunity.
Young kids’ COVID vaccines create approval dilemma for FDATwo doses of Pfizer's vaccine showed mixed results in trials, but the agency is considering approving it anyways in anticipation that a future third shot will provide children with strong immunity.
Read more »
 FDA delays public meeting for COVID-19 vaccine for children under 5 to review more dataBREAKING: The FDA has delayed a public meeting for a COVID-19 vaccine for children under 5 in order to review more data.
FDA delays public meeting for COVID-19 vaccine for children under 5 to review more dataBREAKING: The FDA has delayed a public meeting for a COVID-19 vaccine for children under 5 in order to review more data.
Read more »
 FDA delays public meeting for COVID-19 vaccine for children under 5 to review more dataThe FDA has delayed a public meeting for a COVID-19 vaccine for children under 5 in order to review more data.
FDA delays public meeting for COVID-19 vaccine for children under 5 to review more dataThe FDA has delayed a public meeting for a COVID-19 vaccine for children under 5 in order to review more data.
Read more »
 FDA delays public meeting for COVID-19 vaccine for children under 5 to review more dataThe FDA has delayed a public meeting for a COVID-19 vaccine for children under 5 in order to review more data.
FDA delays public meeting for COVID-19 vaccine for children under 5 to review more dataThe FDA has delayed a public meeting for a COVID-19 vaccine for children under 5 in order to review more data.
Read more »