The Food and Drug Administration on Monday cleared the Pfizer-BioNTech Covid-19 booster dose for children ages 12 to 15.
The decreased time period to wait for a booster only applies to those who had the Pfizer shots. Those who received the Moderna shots should still wait six months before getting the booster, the FDA said, citing a lack of data from Moderna to change the recommended time period.Boosters for Johnson & Johnson are given after two months.
The director of the FDA's Center for Biologics Evaluation and Research, Dr. Peter Marks, predicted it will take"weeks to months" for the agency to sort through data on mixing the Johnson & Johnson vaccine with third doses of one of the mRNA vaccines. "Those data are becoming available from those who receive complex combinations in Europe and elsewhere," Marks said."As we get those data, we'll analyze them and then potentially make recommendations."
The change in timing for Pfizer's booster comes from studies of more than 6,300 teens ages 12 to 15 in Israel. That research suggested that a Pfizer booster dose may protect them from the omicron variant better when given at five months, rather than six. "With the current wave of the omicron variant, it's critical that we continue to take effective, life-saving preventative measures such as primary vaccination and boosters, mask wearing and social distancing in order to effectively fight Covid-19," acting FDA Commissioner Dr.
Canada Latest News, Canada Headlines
Similar News:You can also read news stories similar to this one that we have collected from other news sources.
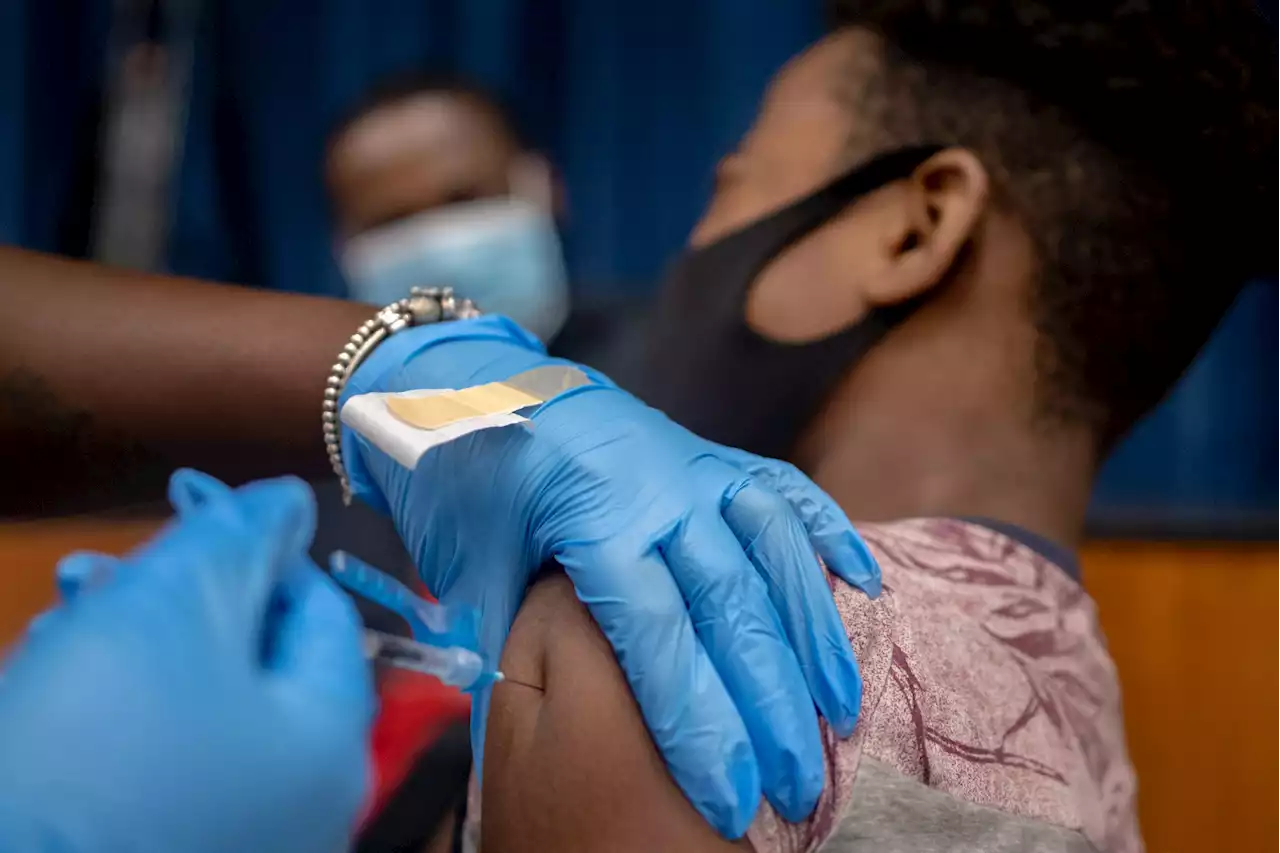 FDA authorizes Pfizer Covid-19 vaccine boosters for 12- to 15-year-oldsThe US kicked off 2022 amid a massive Covid-19 case spike — driven by the highly contagious Omicron variant — that some experts warn will be different than any other time in the pandemic.
FDA authorizes Pfizer Covid-19 vaccine boosters for 12- to 15-year-oldsThe US kicked off 2022 amid a massive Covid-19 case spike — driven by the highly contagious Omicron variant — that some experts warn will be different than any other time in the pandemic.
Read more »
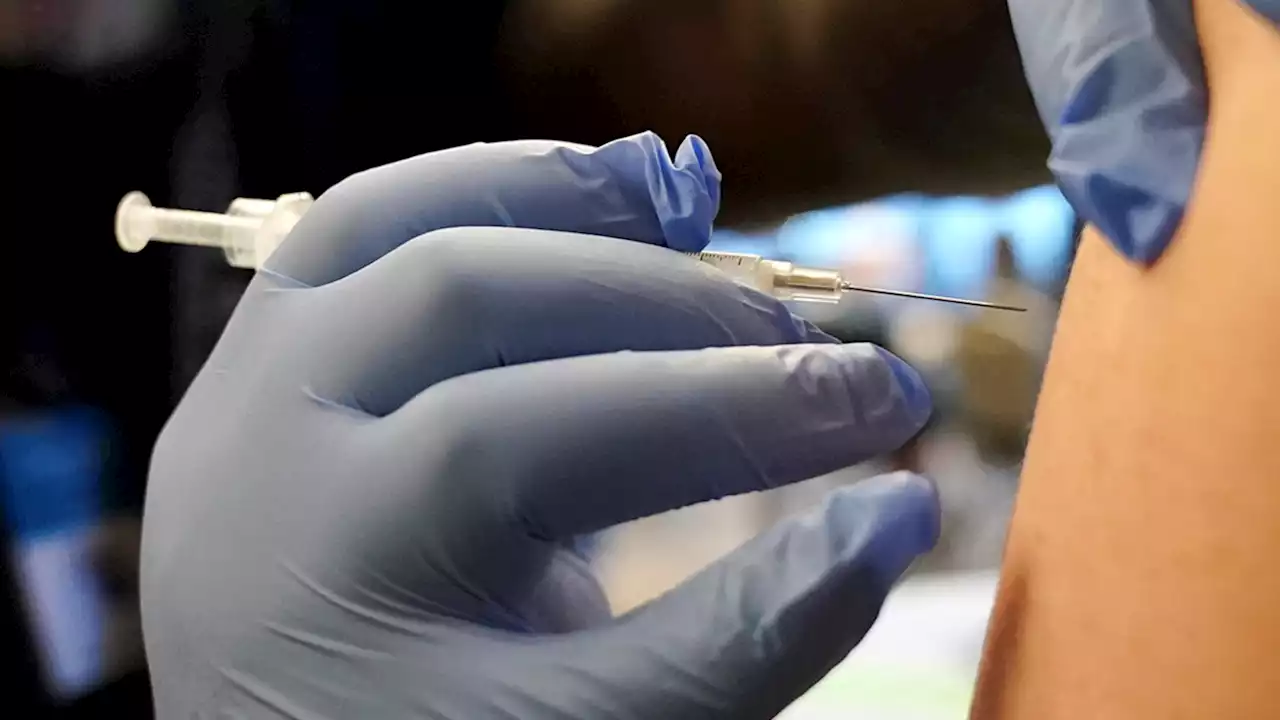 FDA expands Pfizer COVID-19 boosters for 12- to 15-year-olds as omicron surgesThe U.S. is expanding COVID-19 boosters as it confronts the omicron surge, with the FDA allowing extra Pfizer shots for children as young as 12.
FDA expands Pfizer COVID-19 boosters for 12- to 15-year-olds as omicron surgesThe U.S. is expanding COVID-19 boosters as it confronts the omicron surge, with the FDA allowing extra Pfizer shots for children as young as 12.
Read more »
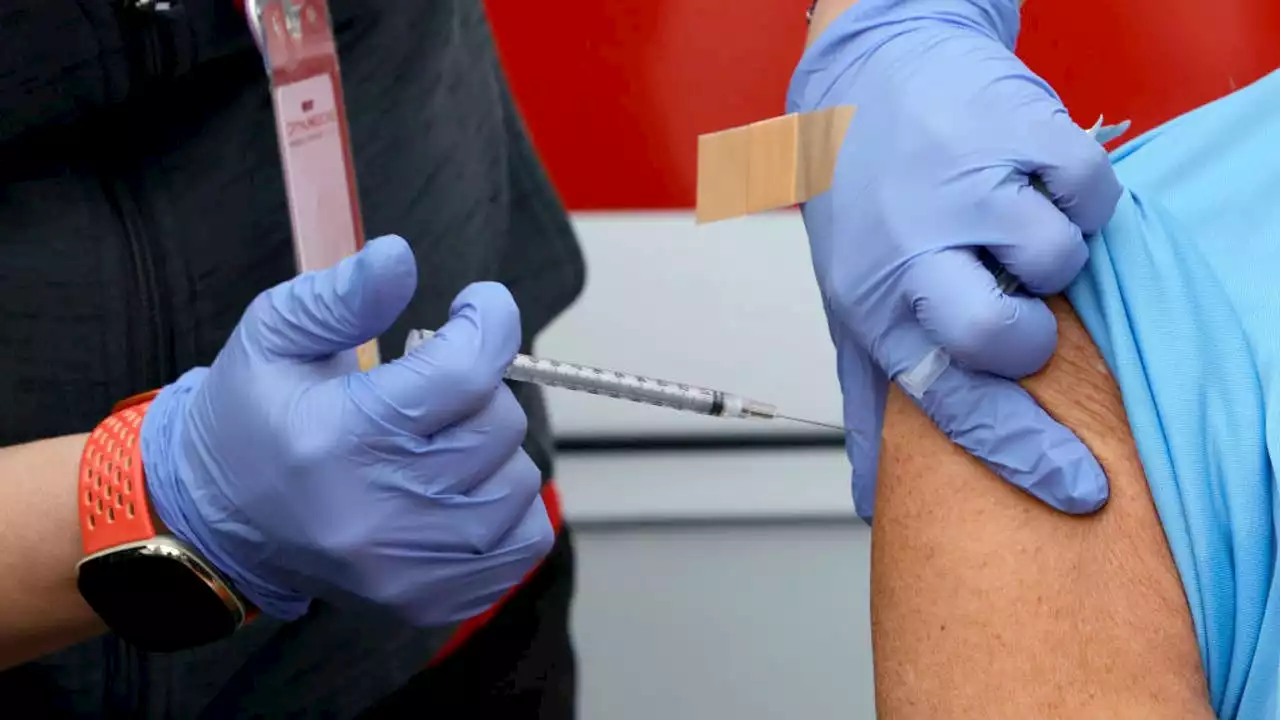 FDA expands Pfizer COVID-19 vaccine booster for ages 12 to 15The FDA expanded Pfizer’s COVID-19 vaccine booster for kids as young as 12. Final approval from the CDC is still needed.
FDA expands Pfizer COVID-19 vaccine booster for ages 12 to 15The FDA expanded Pfizer’s COVID-19 vaccine booster for kids as young as 12. Final approval from the CDC is still needed.
Read more »
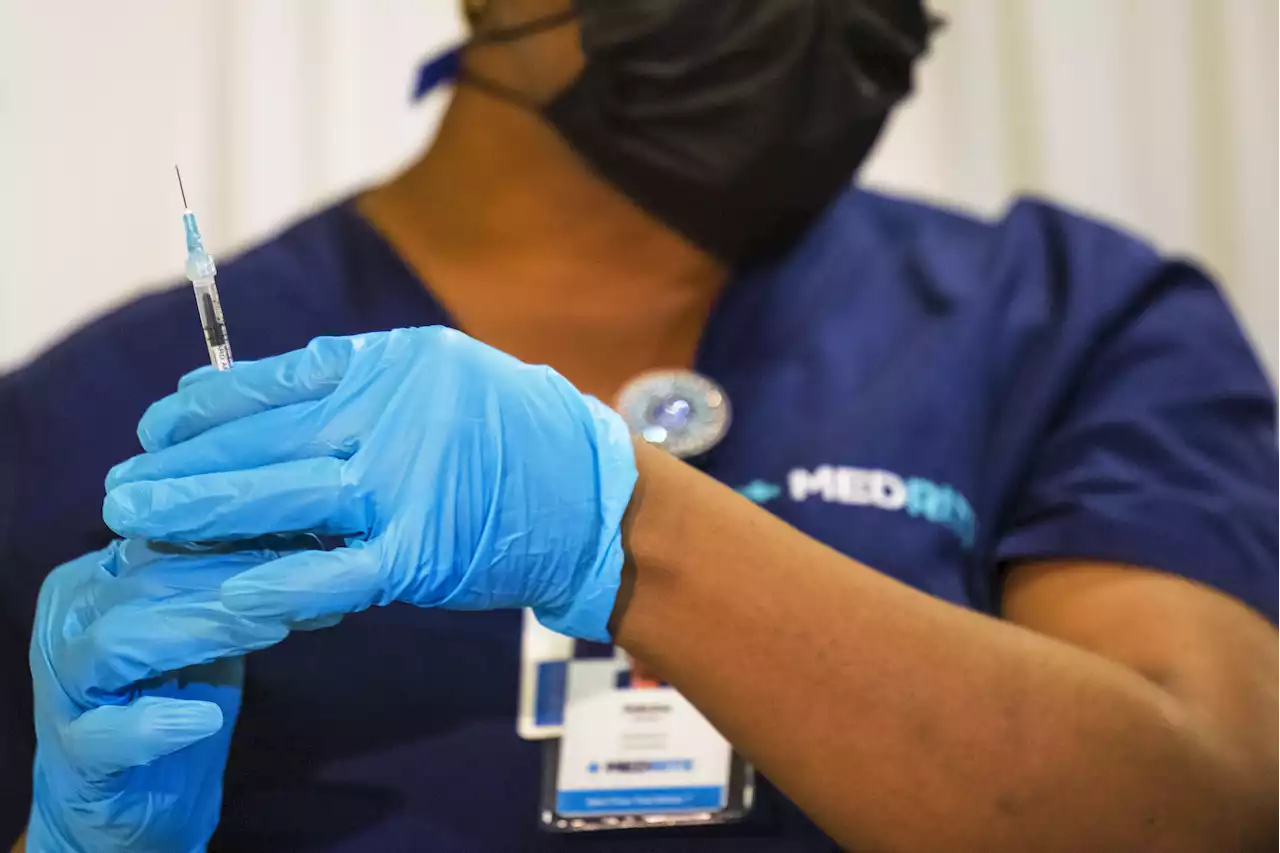 FDA approves Pfizer's COVID booster for kids ages 12 to 15The CDC still needs to rule in favor of the booster in order for children around the U.S. to receive boosters.
FDA approves Pfizer's COVID booster for kids ages 12 to 15The CDC still needs to rule in favor of the booster in order for children around the U.S. to receive boosters.
Read more »
 FDA authorizes Covid boosters for teens 12-15The agency will also allow some immunocompromised children as young as age 5 to get an additional dose.
FDA authorizes Covid boosters for teens 12-15The agency will also allow some immunocompromised children as young as age 5 to get an additional dose.
Read more »
