Researchers unravel the crystal structure of a key enzyme of SARS-CoV-2, paving the way for new antivirals. A high-resolution crystal structure of an enzyme essential to the survival of SARS-CoV-2, the virus that causes COVID-19 has been produced by a team of Mount Sinai researchers. The discover
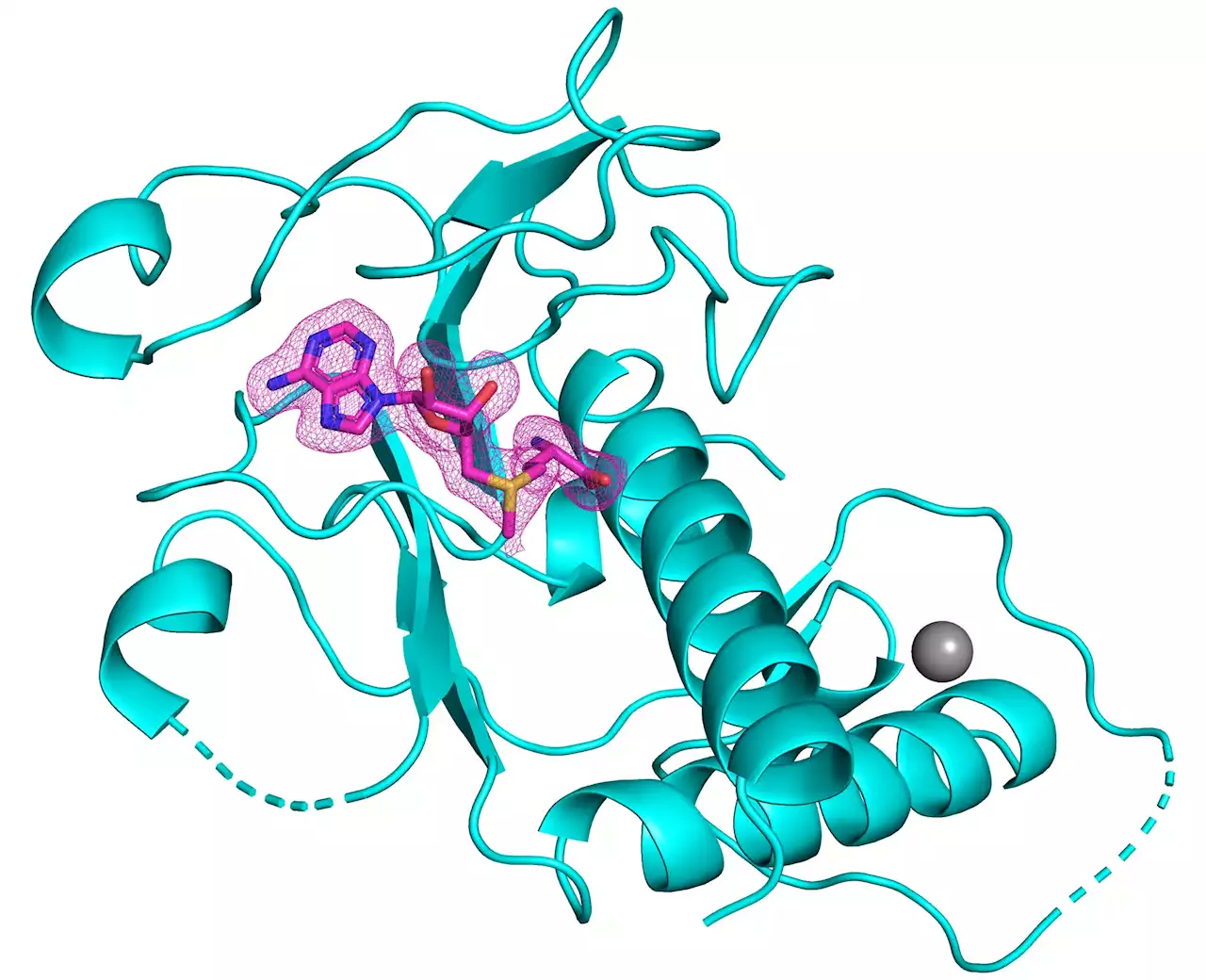
3-D structure of SARS-CoV-2 nsp14 methyltransferase domain bound to its natural cofactor S-adenosylmethionine . Credit: Kottur, et al; Nature Structural & Molecular Biology, paving the way for new antivirals.
“Being able to visualize the shape of the methyltransferase domain of nsp14 at high resolution gives us insights into how to design small molecules that fit into its active site, and thus inhibit its essential chemistry,” says senior author Aneel Aggarwal, PhD. He is Professor of Pharmacological Sciences at the Icahn School of Medicine at Mount Sinai.
“Part of what drives our work,” says Dr. Aggarwal, “is the knowledge gained from treating HIV—that you typically need a cocktail of inhibitors for maximum impact against the virus.” Making the discovery possible was the ability of scientists to clear a hurdle that had prevented others in the past from creating three-dimensional crystals of the nsp14 methytransferase domain. “We employed an approach known as fusion-assisted crystallization,” explains lead author Jithesh Kottur, PhD. He is a postdoctoral fellow at Icahn Mount Sinai, and a crystallographer and biochemist. “It involves fusing the enzyme with another small protein that helps it to crystalize.