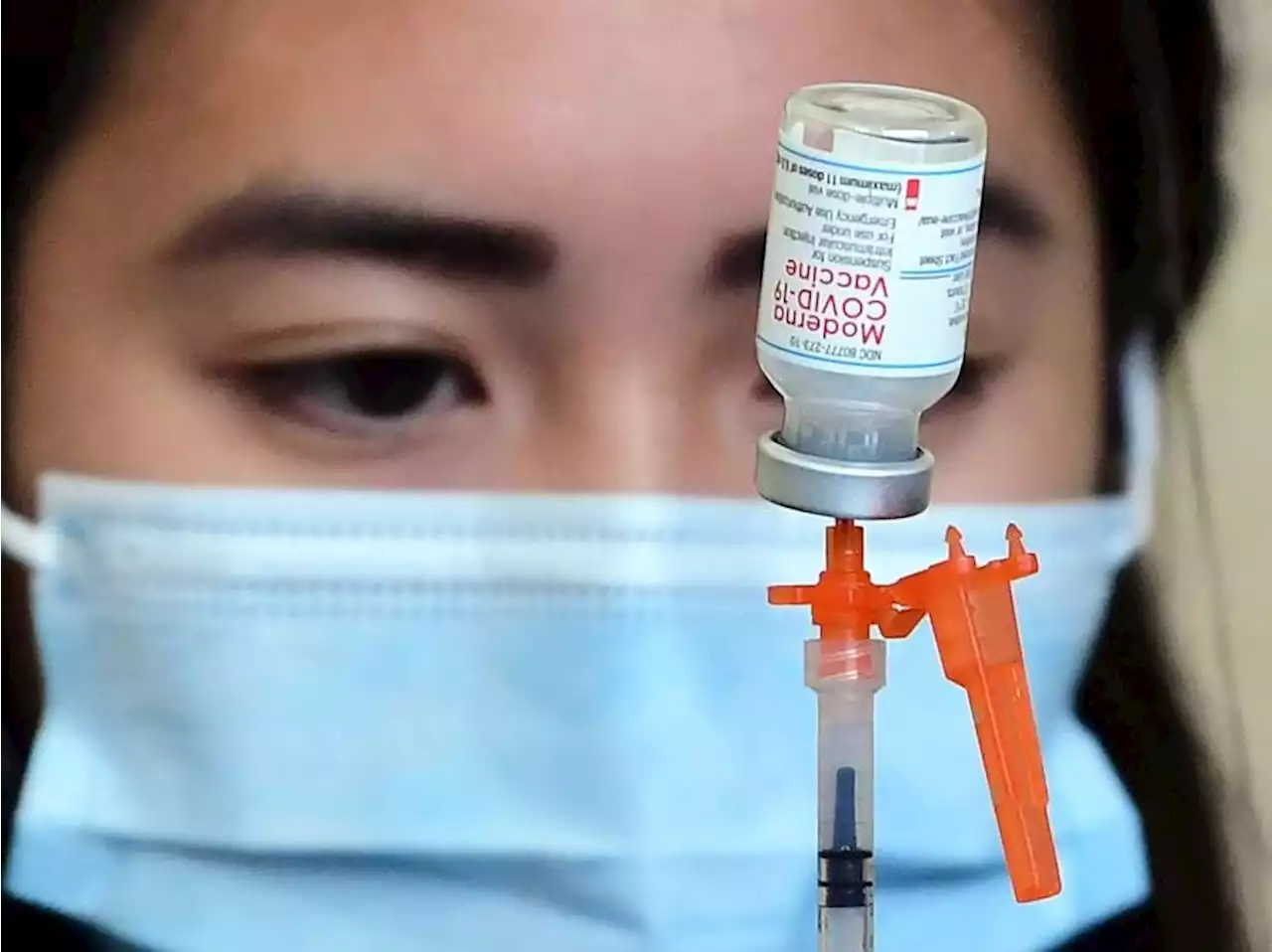
Britain has given the first green light to a variant\u002Dadapted COVID\u002D19 shot that targets both the original and Omicron version of the virus.
The U.K. medicines regulator gave the so-called bivalent vaccine made by U.S. drug company Moderna conditional approval as a booster for adults on Monday.
Moderna in June said trial data showed that when given as a fourth dose, the variant-adapted shot raised virus-neutralizing antibodies by eight-fold against Omicron. No serious safety concerns were identified with the new Moderna formulation, the MHRA added on Monday. “The first generation of COVID-19 vaccines being used in the U.K. continue to provide important protection against the disease and save lives,” MHRA Chief Executive June Raine said in a statement.Article content
In contrast, the U.S. Food and Drug Administration has said it will seek the specific inclusion of the newer BA.4 and BA.5 offshoots of Omicron in any new shots used domestically.
Canada Latest News, Canada Headlines
Similar News:You can also read news stories similar to this one that we have collected from other news sources.
 Britain first country to approve variant-adapted COVID vaccineModerna\u0027s bivalent vaccine targets the original 2020 COVID virus and Omicron variant
Britain first country to approve variant-adapted COVID vaccineModerna\u0027s bivalent vaccine targets the original 2020 COVID virus and Omicron variant
Read more »
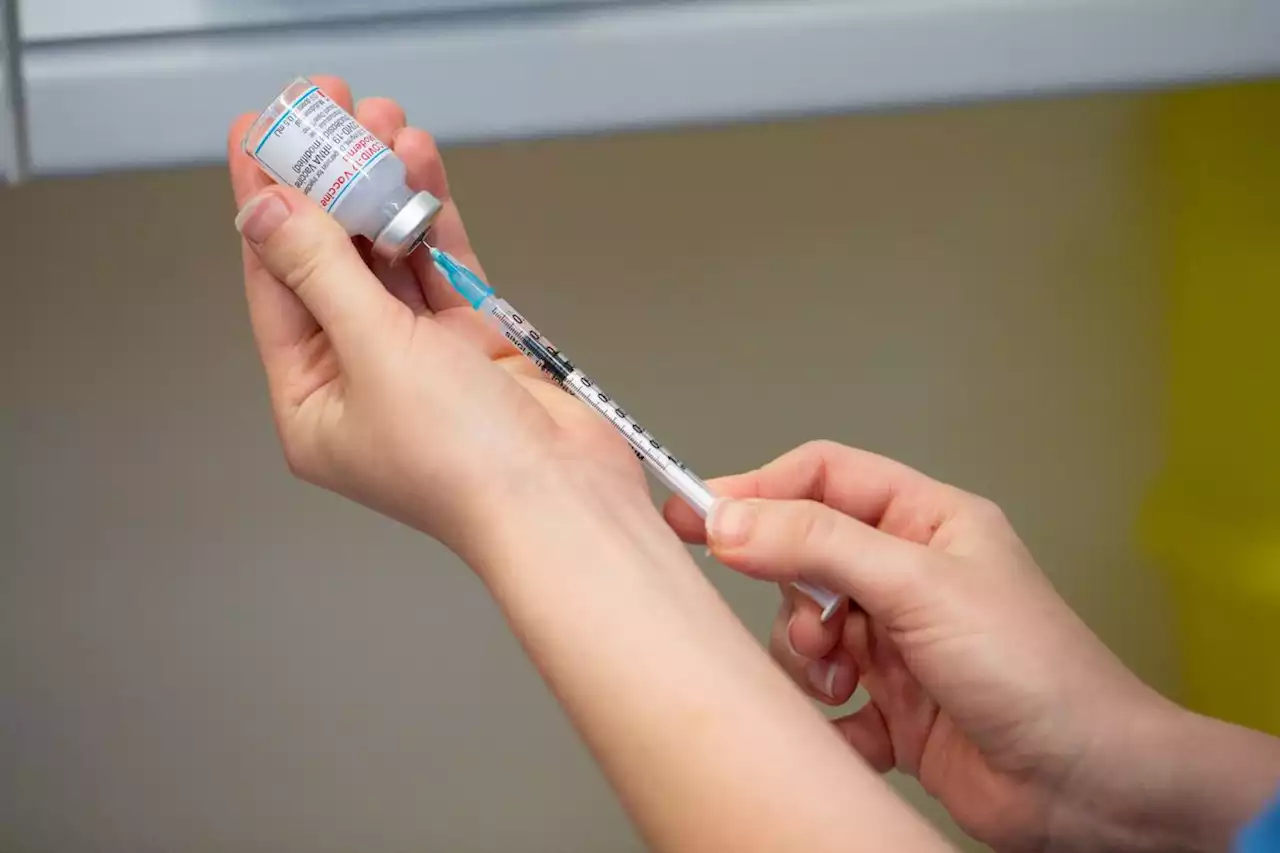 Britain becomes first country to approve a bivalent COVID-19 vaccine boosterThe bivalent vaccine, made by U.S. drug company Moderna, targets both the original and Omicron variants of the virus
Britain becomes first country to approve a bivalent COVID-19 vaccine boosterThe bivalent vaccine, made by U.S. drug company Moderna, targets both the original and Omicron variants of the virus
Read more »
 UK becomes first country to approve updated COVID-19 vaccine which tackles both original virus and Omicron variantModerna’s updated vaccine contains a half dose of its original shot against the initial virus and another half targeting an earlier version of the Omicron variant.
UK becomes first country to approve updated COVID-19 vaccine which tackles both original virus and Omicron variantModerna’s updated vaccine contains a half dose of its original shot against the initial virus and another half targeting an earlier version of the Omicron variant.
Read more »
 Pfizer CEO tests positive for COVID, says has very mild symptomsPfizer Inc Chief Executive Officer Albert Bourla said on Monday he had tested positive for COVID\u002D19 and was experiencing very mild symptoms.
Pfizer CEO tests positive for COVID, says has very mild symptomsPfizer Inc Chief Executive Officer Albert Bourla said on Monday he had tested positive for COVID\u002D19 and was experiencing very mild symptoms.
Read more »
 Pfizer CEO tests positive for COVID, says has very mild symptomsThe Pfizer CEO says he\u0027s had four doses of the vaccine and is taking the company\u0027s oral COVID\u002D19 antiviral treatment, Paxlovid
Pfizer CEO tests positive for COVID, says has very mild symptomsThe Pfizer CEO says he\u0027s had four doses of the vaccine and is taking the company\u0027s oral COVID\u002D19 antiviral treatment, Paxlovid
Read more »