The FDA is under fire for allegedly caving to political pressure to ban vapes and e-cigarettes while simultaneously allowing Chinese products to flood the U.S. market.
to prevent ENDS from going to market. For example, several Democrats in Congress have pushed the agency to deny the applications of Juul Labs Inc., an e-cigarette company, and remove its products from the market.
The day after that, Rep. Raja Krishnamoorthi, D-Ill., said he was"heartened" by the FDA's decisions following a"long conversation" he had with agency officials. He added he's"glad that at least we have an ally in the FDA commissioner." Months later, the drug-focused publication Filter reported that internal FDA memos showed that the CTP's Office of Science had actually recommended to authorize menthol-flavored vaping products as permissible to go to market. However,, the office later reversed course due to pressure from agency leadership after the office of CTP Director Brian King intervened.
A product is deemed permissible to go to market and appropriate for public health based largely on the likelihood of it transitioning adults away from cigarettes without introducing a new generation to nicotine.
Canada Latest News, Canada Headlines
Similar News:You can also read news stories similar to this one that we have collected from other news sources.
 FDA advisors recommend AstraZeneca antibody to protect babies from RSVIf the Food and Drug Administration approves nirsevimab, it will be the first medical intervention available in the U.S. that can protect infants from RSV.
FDA advisors recommend AstraZeneca antibody to protect babies from RSVIf the Food and Drug Administration approves nirsevimab, it will be the first medical intervention available in the U.S. that can protect infants from RSV.
Read more »
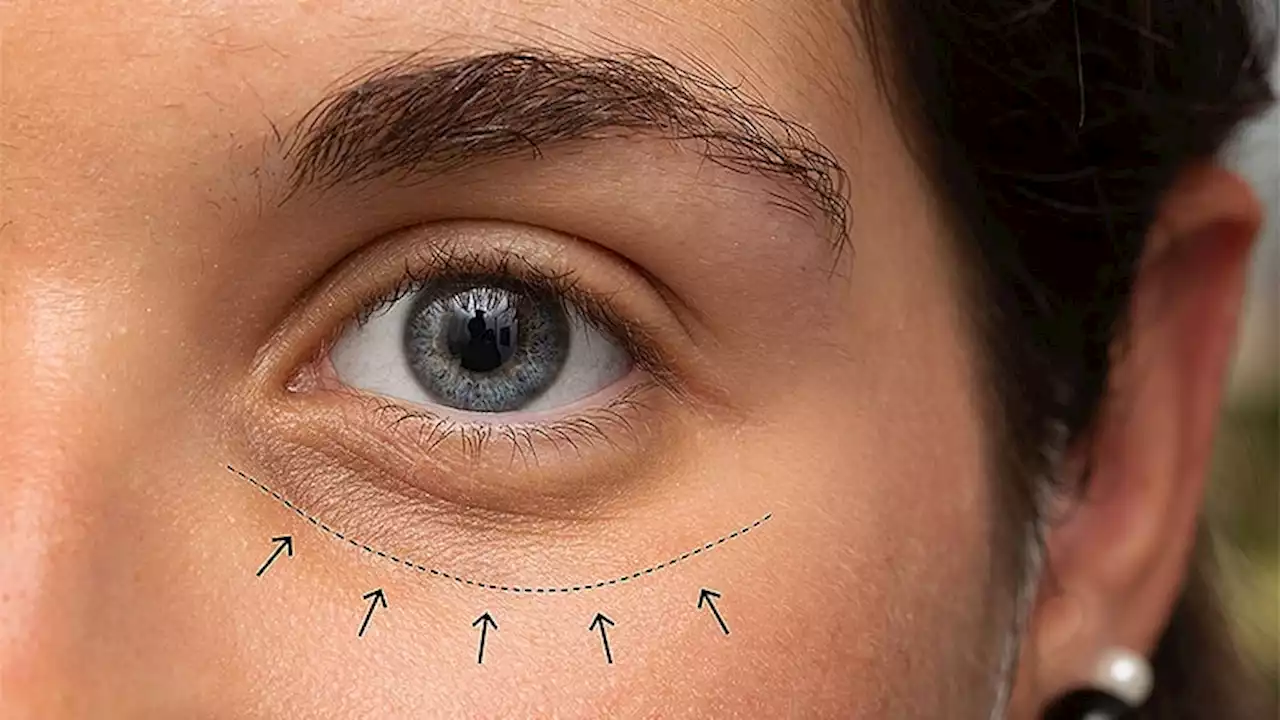 FDA Approves Hyaluronic Acid Filler for Under-Eye HollowsIn the phase 3 study that was the basis of approval, 87% of those treated showed improvement in under-eye hollows at 3 months.
FDA Approves Hyaluronic Acid Filler for Under-Eye HollowsIn the phase 3 study that was the basis of approval, 87% of those treated showed improvement in under-eye hollows at 3 months.
Read more »
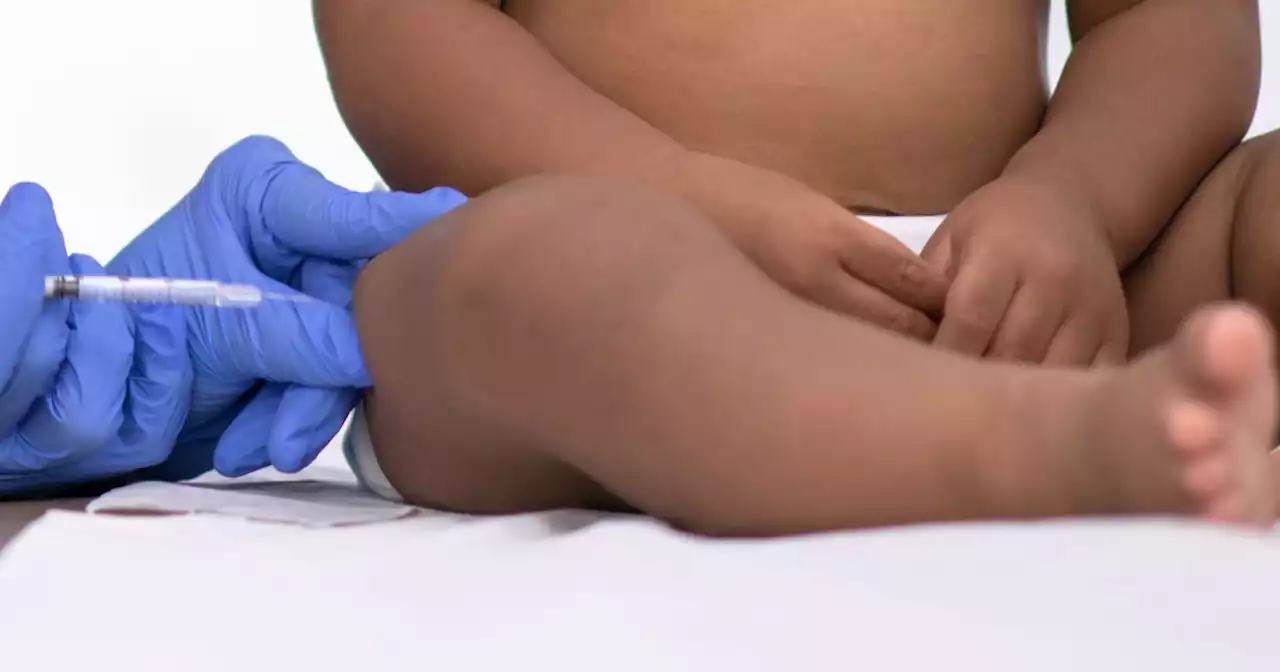 FDA panel recommends injectable drug to prevent RSV in infantsThe monoclonal antibody shot would be given to babies during or before their first RSV season, as well as to high-risk children up to age 2 entering their second RSV season.
FDA panel recommends injectable drug to prevent RSV in infantsThe monoclonal antibody shot would be given to babies during or before their first RSV season, as well as to high-risk children up to age 2 entering their second RSV season.
Read more »
 US FDA panel backs Sanofi-AstraZeneca's preventive RSV therapyThe U.S. Food and Drug Administration advisers on Thursday backed the use of Sanofi and partner AstraZeneca's experimental antibody to prevent respiratory syncytial virus (RSV) infections in infants.
US FDA panel backs Sanofi-AstraZeneca's preventive RSV therapyThe U.S. Food and Drug Administration advisers on Thursday backed the use of Sanofi and partner AstraZeneca's experimental antibody to prevent respiratory syncytial virus (RSV) infections in infants.
Read more »
 Eisai-Biogen Alzheimer's Drug Data Confirms Benefits: FDAUS Food and Drug Administration staff on Wednesday said data from a late-stage trial of Eisai and Biogen's Alzheimer's disease drug suggests it offered a meaningful benefit to patients.
Eisai-Biogen Alzheimer's Drug Data Confirms Benefits: FDAUS Food and Drug Administration staff on Wednesday said data from a late-stage trial of Eisai and Biogen's Alzheimer's disease drug suggests it offered a meaningful benefit to patients.
Read more »
